Avoiding a reproducibility crisis in regulatory toxicology-on the fundamental role of ring trials
- PMID: 38689008
- PMCID: PMC11169035
- DOI: 10.1007/s00204-024-03736-z
Avoiding a reproducibility crisis in regulatory toxicology-on the fundamental role of ring trials
Abstract
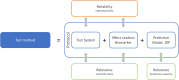
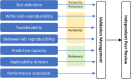
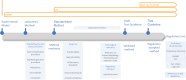
The ongoing transition from chemical hazard and risk assessment based on animal studies to assessment relying mostly on non-animal data, requires a multitude of novel experimental methods, and this means that guidance on the validation and standardisation of test methods intended for international applicability and acceptance, needs to be updated. These so-called new approach methodologies (NAMs) must be applicable to the chemical regulatory domain and provide reliable data which are relevant to hazard and risk assessment. Confidence in and use of NAMs will depend on their reliability and relevance, and both are thoroughly assessed by validation. Validation is, however, a time- and resource-demanding process. As updates on validation guidance are conducted, the valuable components must be kept: Reliable data are and will remain fundamental. In 2016, the scientific community was made aware of the general crisis in scientific reproducibility-validated methods must not fall into this. In this commentary, we emphasize the central importance of ring trials in the validation of experimental methods. Ring trials are sometimes considered to be a major hold-up with little value added to the validation. Here, we clarify that ring trials are indispensable to demonstrate the robustness and reproducibility of a new method. Further, that methods do fail in method transfer and ring trials due to different stumbling blocks, but these provide learnings to ensure the robustness of new methods. At the same time, we identify what it would take to perform ring trials more efficiently, and how ring trials fit into the much-needed update to the guidance on the validation of NAMs.
Keywords: OECD Test Guidelines; Reliability; Ring trials; Robustness; Validation.
© 2024. The Author(s).
Figures
Similar articles
-
Validation of Alternative In Vitro Methods to Animal Testing: Concepts, Challenges, Processes and Tools.Adv Exp Med Biol. 2016;856:65-132. doi: 10.1007/978-3-319-33826-2_4. Adv Exp Med Biol. 2016. PMID: 27671720 Review.
-
The benefits of validation of methods for toxicity testing outweigh its costs.ALTEX. 2024;41(3):395-401. doi: 10.14573/altex.2403051. Epub 2024 Mar 19. ALTEX. 2024. PMID: 38501278
-
Application of omics data in regulatory toxicology: report of an international BfR expert workshop.Arch Toxicol. 2015 Nov;89(11):2177-84. doi: 10.1007/s00204-015-1602-x. Epub 2015 Oct 20. Arch Toxicol. 2015. PMID: 26486796
-
Workshop on acceleration of the validation and regulatory acceptance of alternative methods and implementation of testing strategies.Toxicol In Vitro. 2018 Aug;50:62-74. doi: 10.1016/j.tiv.2018.02.018. Epub 2018 Mar 2. Toxicol In Vitro. 2018. PMID: 29501630
-
New approach methodologies (NAMs) for human-relevant biokinetics predictions. Meeting the paradigm shift in toxicology towards an animal-free chemical risk assessment.ALTEX. 2020;37(4):607-622. doi: 10.14573/altex.2003242. Epub 2020 Jun 8. ALTEX. 2020. PMID: 32521035
Cited by
-
A hair-follicle reconstructed in vitro immunocompetent skin model for prediction of the sensitizing potential of chemicals.Arch Toxicol. 2025 Jul 18. doi: 10.1007/s00204-025-04130-z. Online ahead of print. Arch Toxicol. 2025. PMID: 40681688
-
Addressing chemically-induced obesogenic metabolic disruption: selection of chemicals for in vitro human PPARα, PPARγ transactivation, and adipogenesis test methods.Front Endocrinol (Lausanne). 2024 Jul 8;15:1401120. doi: 10.3389/fendo.2024.1401120. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39040675 Free PMC article. Review.
-
A 2024 inventory of test methods relevant to thyroid hormone system disruption for human health and environmental regulatory hazard assessment.Open Res Eur. 2024 Nov 6;4:242. doi: 10.12688/openreseurope.18739.1. eCollection 2024. Open Res Eur. 2024. PMID: 39931575 Free PMC article. Review.
-
Human health risk assessment for microbial pesticides in the EU: challenges and perspectives.Environ Health. 2025 Jul 2;24(1):43. doi: 10.1186/s12940-025-01196-1. Environ Health. 2025. PMID: 40605039 Free PMC article. Review.
-
Challenges and Opportunities in the Development and Adoption of New Approach Methods (NAMs).J Agric Food Chem. 2025 Apr 2;73(13):7519-7521. doi: 10.1021/acs.jafc.5c02930. Epub 2025 Mar 19. J Agric Food Chem. 2025. PMID: 40106668 Free PMC article. No abstract available.
References
-
- Bas A, Burns N, Gulotta A, Junker J, Drasler B, Lehner R, Aicher L, Constant S, Fink A, Rothen-Rutishauser B. Understanding the development, standardization, and validation process of alternative in vitro test methods for regulatory approval from a researcher perspective. Small. 2021 doi: 10.1002/smll.202006027. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

