Correlates of protection against symptomatic SARS-CoV-2 in vaccinated children
- PMID: 38689059
- PMCID: PMC11164684
- DOI: 10.1038/s41591-024-02962-3
Correlates of protection against symptomatic SARS-CoV-2 in vaccinated children
Erratum in
-
Publisher Correction: Correlates of protection against symptomatic SARS-CoV-2 in vaccinated children.Nat Med. 2024 Jul;30(7):2093. doi: 10.1038/s41591-024-03107-2. Nat Med. 2024. PMID: 38858526 Free PMC article. No abstract available.
Abstract
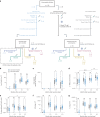
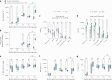
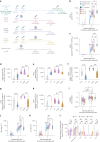
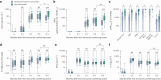
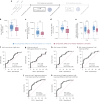
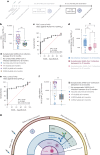
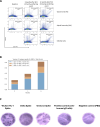
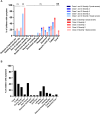
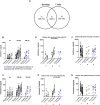
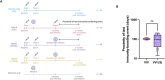
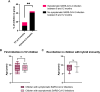
The paucity of information on longevity of vaccine-induced immune responses and uncertainty of the correlates of protection hinder the development of evidence-based COVID-19 vaccination policies for new birth cohorts. Here, to address these knowledge gaps, we conducted a cohort study of healthy 5-12-year-olds vaccinated with BNT162b2. We serially measured binding and neutralizing antibody titers (nAbs), spike-specific memory B cell (MBC) and spike-reactive T cell responses over 1 year. We found that children mounted antibody, MBC and T cell responses after two doses of BNT162b2, with higher antibody and T cell responses than adults 6 months after vaccination. A booster (third) dose only improved antibody titers without impacting MBC and T cell responses. Among children with hybrid immunity, nAbs and T cell responses were highest in those infected after two vaccine doses. Binding IgG titers, MBC and T cell responses were predictive, with T cells being the most important predictor of protection against symptomatic infection before hybrid immunity; nAbs only correlated with protection after hybrid immunity. The stable MBC and T cell responses over time suggest sustained protection against symptomatic SARS-CoV-2 infection, even when nAbs wane. Booster vaccinations do not confer additional immunological protection to healthy children.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
N.L.B. reports a patent for a method to monitor SARS-CoV-2-specific T cells in biological samples, which is pending. The other authors declare no competing interests.
Figures



References
-
- World Health Organization. Interim statement on hybrid immunity and increasing population seroprevalence rates. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-imm... (2022).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

