Fundamentals of redox regulation in biology
- PMID: 38689066
- PMCID: PMC11921270
- DOI: 10.1038/s41580-024-00730-2
Fundamentals of redox regulation in biology
Erratum in
-
Author Correction: Fundamentals of redox regulation in biology.Nat Rev Mol Cell Biol. 2024 Sep;25(9):758. doi: 10.1038/s41580-024-00754-8. Nat Rev Mol Cell Biol. 2024. PMID: 38886589 No abstract available.
Abstract
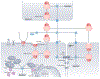
Oxidation-reduction (redox) reactions are central to the existence of life. Reactive species of oxygen, nitrogen and sulfur mediate redox control of a wide range of essential cellular processes. Yet, excessive levels of oxidants are associated with ageing and many diseases, including cardiological and neurodegenerative diseases, and cancer. Hence, maintaining the fine-tuned steady-state balance of reactive species production and removal is essential. Here, we discuss new insights into the dynamic maintenance of redox homeostasis (that is, redox homeodynamics) and the principles underlying biological redox organization, termed the 'redox code'. We survey how redox changes result in stress responses by hormesis mechanisms, and how the lifelong cumulative exposure to environmental agents, termed the 'exposome', is communicated to cells through redox signals. Better understanding of the molecular and cellular basis of redox biology will guide novel redox medicine approaches aimed at preventing and treating diseases associated with disturbed redox regulation.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
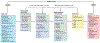


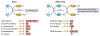
References
-
- Jacob C, Giles GI, Giles NM & Sies H Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl 42, 4742–4758 (2003). - PubMed
-
- Lennicke C & Cochemé HM Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021). - PubMed
-
- Sies H et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol 23, 499–515 (2022). - PubMed
-
This ‘Expert Recommendation’ addresses key questions regarding the impact of oxidants on physiology and their contribution to disease.
-
- Thannickal VJ & Fanburg BL Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol 279, L1005–L1028 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

