Stress-induced epinephrine promotes hepatocellular carcinoma progression via the USP10-PLAGL2 signaling loop
- PMID: 38689092
- PMCID: PMC11148159
- DOI: 10.1038/s12276-024-01223-0
Stress-induced epinephrine promotes hepatocellular carcinoma progression via the USP10-PLAGL2 signaling loop
Abstract
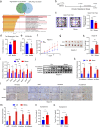
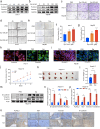
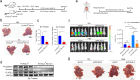
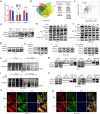
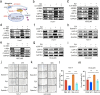
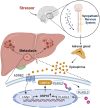
Hepatocellular carcinoma (HCC) is associated with a poor prognosis. Our previous study demonstrated that Pleomorphic adenoma gene like-2 (PLAGL2) was a potential therapeutic target in HCC. However, the mechanisms that lead to the upregulation of PLAGL2 in HCC remain unclear. The present study revealed that stress-induced epinephrine increased the expression of PLAGL2, thereby promoting the progression of HCC. Furthermore, PLAGL2 knockdown inhibited epinephrine-induced HCC development. Mechanistically, epinephrine upregulated ubiquitin-specific protease 10 (USP10) to stabilize PLAGL2 via the adrenergic β-receptor-2-c-Myc (ADRB2-c-Myc) axis. Furthermore, PLAGL2 acted as a transcriptional regulator of USP10, forming a signaling loop. Taken together, these results reveal that stress-induced epinephrine activates the PLAGL2-USP10 signaling loop to enhance HCC progression. Furthermore, PLAGL2 plays a crucial role in psychological stress-mediated promotion of HCC progression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

