Rapid, sensitive, and user-friendly detection of Pseudomonas aeruginosa using the RPA/CRISPR/Cas12a system
- PMID: 38689239
- PMCID: PMC11061930
- DOI: 10.1186/s12879-024-09348-3
Rapid, sensitive, and user-friendly detection of Pseudomonas aeruginosa using the RPA/CRISPR/Cas12a system
Abstract
Background: Pseudomonas aeruginosa (P. aeruginosa) is a life-threatening bacterium known for its rapid development of antibiotic resistance, posing significant challenges in clinical treatment, biosecurity, food safety, and environmental monitoring. Early and accurate identification of P. aeruginosa is crucial for effective intervention.
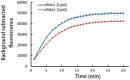
Methods: The lasB gene of P. aeruginosa was selected as the target for the detection. RPA primers for recombinase polymerase amplification (RPA) and crRNA for CRISPR/Cas12a detection were meticulously designed to target specific regions within the lasB gene. The specificity of the RPA/CRISPR/Cas12a detection platform was assessed using 15 strains. The detection limit of RPA/CRISPR/Cas12a detection platform was determined by utilizing a pseudo-dilution series of the P. aeruginosa DNA. The practical applicability of the RPA/CRISPR/Cas12a detection platform was validated by comparing it with qPCR on 150 samples (35 processed meat product samples, 55 cold seasoned vegetable dishes, 60 bottled water samples).
Results: The RPA/CRISPR/Cas12a detection platform demonstrates high specificity, with no cross-reactivity with non-P. aeruginosa strains. This assay exhibits remarkable sensitivity, with a limit of detection (LOD) of 100 copies/µL for fluorescence assay and 101 copies/µL for the LFTS method. Furthermore, the performance of the RPA/CRISPR/Cas12a detection platform is comparable to that of the well-established qPCR method, while offering advantages such as shorter reaction time, simplified operation, and reduced equipment requirements.
Conclusions: The RPA/CRISPR/Cas12a detection platform presents a straightforward, accurate, and sensitive approach for early P. aeruginosa detection and holds great promise for diverse applications requiring rapid and reliable identification.
Keywords: Pseudomonas aeruginosa; CRISPR/Cas12a; Detection; Recombinase polymerase amplification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
The authors declare no competing interests.
Figures




References
-
- Qin SG, Xiao W, Zhou CM, Pu QQ, Deng X, Lan LF, Liang HH, Song XR, Wu M. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi: 10.1038/s41392-022-01056-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

