WNT5B drives osteosarcoma stemness, chemoresistance and metastasis
- PMID: 38689429
- PMCID: PMC11061378
- DOI: 10.1002/ctm2.1670
WNT5B drives osteosarcoma stemness, chemoresistance and metastasis
Abstract
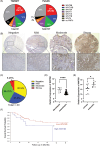
Background: Treatment for osteosarcoma, a paediatric bone cancer with no therapeutic advances in over three decades, is limited by a lack of targeted therapies. Osteosarcoma frequently metastasises to the lungs, and only 20% of patients survive 5 years after the diagnosis of metastatic disease. We found that WNT5B is the most abundant WNT expressed in osteosarcoma tumours and its expression correlates with metastasis, histologic subtype and reduced survival.
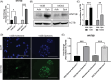
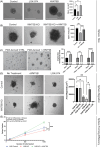
Methods: Using tumor-spheroids to model cancer stem-like cells, we performed qPCR, immunoblotting, and immunofluorescence to monitor changes in gene and protein expression. Additionally, we measured sphere size, migration and forming efficiency to monitor phenotypic changes. Therefore, we characterised WNT5B's relevance to cancer stem-like cells, metastasis, and chemoresistance and evaluated its potential as a therapeutic target.
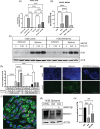
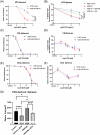
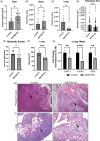
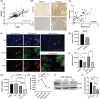
Results: In osteosarcoma cell lines and patient-derived spheres, WNT5B is enriched in stem cells and induces the expression of the stemness gene SOX2. WNT5B promotes sphere size, sphere-forming efficiency, and cell proliferation, migration, and chemoresistance to methotrexate (but not cisplatin or doxorubicin) in spheres formed from conventional cell lines and patient-derived xenografts. In vivo, WNT5B increased osteosarcoma lung and liver metastasis and inhibited the glycosaminoglycan hyaluronic acid via upregulation of hyaluronidase 1 (HYAL1), leading to changes in the tumour microenvironment. Further, we identified that WNT5B mRNA and protein correlate with the receptor ROR1 in primary tumours. Targeting WNT5B through inhibition of WNT/ROR1 signalling with an antibody to ROR1 reduced stemness properties, including chemoresistance, sphere size and SOX2 expression.
Conclusions: Together, these data define WNT5B's role in driving osteosarcoma cancer stem cell expansion and methotrexate resistance and provide evidence that the WNT5B pathway is a promising candidate for treating osteosarcoma patients.
Key points: WNT5B expression is high in osteosarcoma stem cells leading to increased stem cell proliferation and migration through SOX2. WNT5B expression in stem cells increases rates of osteosarcoma metastasis to the lungs and liver in vivo. The hyaluronic acid degradation enzyme HYAL1 is regulated by WNT5B in osteosarcoma contributing to metastasis. Inhibition of WNT5B with a ROR1 antibody decreases osteosarcoma stemness.
Keywords: WNT5B; cancer stem cells; metastasis; osteosarcoma.
© 2024 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Longo‐Sorbello GS, Bertino JR. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86:121‐127. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
