Clinical heterogeneity within the ALS-FTD spectrum in a family with a homozygous optineurin mutation
- PMID: 38689506
- PMCID: PMC11187959
- DOI: 10.1002/acn3.52075
Clinical heterogeneity within the ALS-FTD spectrum in a family with a homozygous optineurin mutation
Abstract
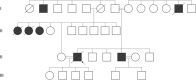
Objective: Mutations in the gene encoding for optineurin (OPTN) have been reported in the context of different neurodegenerative diseases including the amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) spectrum. Based on single case reports, neuropathological data in OPTN mutation carriers have revealed transactive response DNA-binding protein 43 kDa (TDP-43) pathology, in addition to accumulations of tau and alpha-synuclein. Herein, we present two siblings from a consanguineous family with a homozygous frameshift mutation in the OPTN gene and different clinical presentations.
Methods: Both affected siblings underwent (i) clinical, (ii) neurophysiological, (iii) neuropsychological, (iv) radiological, and (v) laboratory examinations, and (vi) whole-exome sequencing (WES). Postmortem histopathological examination was conducted in the index patient, who deceased at the age of 41.
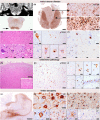
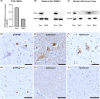
Results: The index patient developed rapidly progressing clinical features of upper and lower motor neuron dysfunction as well as apathy and cognitive deterioration at the age of 41. Autopsy revealed an ALS-FTLD pattern associated with prominent neuronal and oligodendroglial TDP-43 pathology, and an atypical limbic 4-repeat tau pathology reminiscent of argyrophilic grain disease. The brother of the index patient exhibited behavioral changes and mnestic deficits at the age of 38 and was diagnosed with behavioral FTD 5 years later, without any evidence of motor neuron dysfunction. WES revealed a homozygous frameshift mutation in the OPTN gene in both siblings (NM_001008212.2: c.1078_1079del; p.Lys360ValfsTer18).
Interpretation: OPTN mutations can be associated with extensive TDP-43 pathology and limbic-predominant tauopathy and present with a heterogeneous clinical phenotype within the ALS-FTD spectrum within the same family.
© 2024 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Maruyama H, Morino H, Ito H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223‐226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

