Metabolomic analysis of dietary-restriction-induced attenuation of sarcopenia in prematurely aging DNA repair-deficient mice
- PMID: 38689513
- PMCID: PMC11154776
- DOI: 10.1002/jcsm.13433
Metabolomic analysis of dietary-restriction-induced attenuation of sarcopenia in prematurely aging DNA repair-deficient mice
Abstract
Background: Sarcopenia is characterized by loss of skeletal muscle mass and function, and is a major risk factor for disability and independence in the elderly. Effective medication is not available. Dietary restriction (DR) has been found to attenuate aging and aging-related diseases, including sarcopenia, but the mechanism of both DR and sarcopenia are incompletely understood.
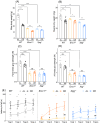
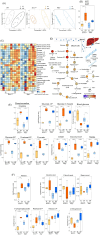
Methods: In this study, mice body weight, fore and all limb grip strength, and motor learning and coordination performance were first analysed to evaluate the DR effects on muscle functioning. Liquid chromatography-mass spectrometry (LC-MS) was utilized for the metabolomics study of the DR effects on sarcopenia in progeroid DNA repair-deficient Ercc1∆/- and Xpg-/- mice, to identify potential biomarkers for attenuation of sarcopenia.
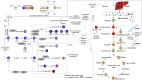
Results: Muscle mass was significantly (P < 0.05) decreased (13-20%) by DR; however, the muscle quality was improved with retained fore limbs and all limbs grip strength in Ercc1∆/- and Xpg-/- mice. The LC-MS results revealed that metabolites and pathways related to oxidative-stress, that is, GSSG/GSH (P < 0.01); inflammation, that is, 9-HODE, 11-HETE (P < 0.05), PGE2, PGD2, and TXB2 (P < 0.01); and muscle growth (PGF2α) (P < 0.01) and regeneration stimulation (PGE2) (P < 0.05) are significantly downregulated by DR. On the other hand, anti-inflammatory indicator and several related metabolites, that is, β-hydroxybutyrate (P < 0.01), 14,15-DiHETE (P < 0.0001), 8,9-EET, 12,13-DiHODE, and PGF1 (P < 0.05); consumption of sources of energy (i.e., muscle and liver glycogen); and energy production pathways, that is, glycolysis (glucose, glucose-6-P, fructose-6-P) (P < 0.01), tricarboxylic acid cycle (succinyl-CoA, malate) (P < 0.001), and gluconeogenesis-related metabolite, alanine (P < 0.01), are significantly upregulated by DR. The notably (P < 0.01) down-modulated muscle growth (PGF2α) and regeneration (PGE2) stimulation metabolite and the increased consumption of glycogen in muscle and liver may be related to the significantly (P < 0.01) lower body weight and muscle mass by DR. The downregulated oxidative stress, pro-inflammatory mediators, and upregulated anti-inflammatory metabolites resulted in a lower energy expenditure, which contributed to enhanced muscle quality together with upregulated energy production pathways by DR. The improved muscle quality may explain why grip strength is maintained and motor coordination and learning performance are improved by DR in Ercc1∆/- and Xpg-/- mice.
Conclusions: This study provides fundamental supporting information on biomarkers and pathways related to the attenuation of sarcopenia, which might facilitate its diagnosis, prevention, and clinical therapy.
Keywords: Dietary restriction; Energy generation; Inflammation; Muscle aging; Oxidative stress; Progeria.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

