Nanomedicine-based disulfiram and metal ion co-delivery strategies for cancer treatment
- PMID: 38689600
- PMCID: PMC11059435
- DOI: 10.1016/j.ijpx.2024.100248
Nanomedicine-based disulfiram and metal ion co-delivery strategies for cancer treatment
Abstract
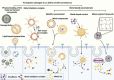
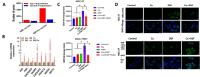
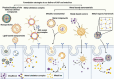
Disulfiram (DSF) is a second-line drug for the clinical treatment of alcoholism and has long been proven to be safe for use in clinical practice. In recent years, researchers have discovered the cancer-killing activity of DSF, which is highly dependent on the presence of metal ions, particularly copper ions. Additionally, free DSF is highly unstable and easily degraded within few minutes in blood circulation. Therefore, an ideal DSF formulation should facilitate the co-delivery of metal ions and safeguard the DSF throughout its biological journey before reaching the targeted site. Extensive research have proved that nanotechnology based formulations can effectively realize this goal by strategic encapsulation therapeutic agents within nanoparticle. To be more specific, this is accomplished through precise delivery, coordinated release of metal ions at the tumor site, thereby amplifying its cytotoxic potential. Beyond traditional co-loading techniques, innovative approaches such as DSF-metal complex and metal nanomaterials, have also demonstrated promising results at the animal model stage. This review aims to elucidate the anticancer mechanism associated with DSF and its reliance on metal ions, as well as to provide a comprehensive overview of recent advances in the arena of nanomedicine based co-delivery strategies for DSF and metal ion in the context of cancer therapy.
Keywords: Co-delivery; Disulfiram; Metal ion; Nanomedicine; cancer treatment.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Disulfiram-loaded metal organic framework for precision cancer treatment via ultrasensitive tumor microenvironment-responsive copper chelation and radical generation.J Colloid Interface Sci. 2022 Jun;615:517-526. doi: 10.1016/j.jcis.2022.01.187. Epub 2022 Feb 3. J Colloid Interface Sci. 2022. PMID: 35152072
-
Nose-to-brain delivery of disulfiram nanoemulsion in situ gel formulation for glioblastoma targeting therapy.Int J Pharm. 2021 Mar 15;597:120250. doi: 10.1016/j.ijpharm.2021.120250. Epub 2021 Jan 22. Int J Pharm. 2021. PMID: 33486040
-
Recent Advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents.Front Mol Biosci. 2021 Sep 17;8:741316. doi: 10.3389/fmolb.2021.741316. eCollection 2021. Front Mol Biosci. 2021. PMID: 34604310 Free PMC article. Review.
-
Old wine in new bottles: Advanced drug delivery systems for disulfiram-based cancer therapy.J Control Release. 2020 Mar 10;319:352-359. doi: 10.1016/j.jconrel.2020.01.001. Epub 2020 Jan 3. J Control Release. 2020. PMID: 31911155 Review.
-
Disulfiram-loaded copper sulfide nanoparticles for potential anti-glioma therapy.Int J Pharm. 2021 Sep 25;607:120978. doi: 10.1016/j.ijpharm.2021.120978. Epub 2021 Aug 8. Int J Pharm. 2021. PMID: 34371152
Cited by
-
M1 macrophage-membrane-cloaked paclitaxel/β-elemene nanoparticles targeting cervical cancer for enhanced therapy.Int J Pharm X. 2024 Aug 13;8:100276. doi: 10.1016/j.ijpx.2024.100276. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39263001 Free PMC article.
-
Insights into microscopic fabrication, macroscopic forms and biomedical applications of alginate composite gel containing metal-organic frameworks.Asian J Pharm Sci. 2024 Dec;19(6):100952. doi: 10.1016/j.ajps.2024.100952. Epub 2024 Sep 1. Asian J Pharm Sci. 2024. PMID: 39640058 Free PMC article. Review.
-
Advanced applications of smart electrospun nanofibers in cancer therapy: With insight into material capabilities and electrospinning parameters.Int J Pharm X. 2024 Jun 26;8:100265. doi: 10.1016/j.ijpx.2024.100265. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39045009 Free PMC article. Review.
-
Exploring disulfiram mechanisms in renal fibrosis: insights from biological data and computational approaches.Front Pharmacol. 2025 Mar 18;16:1480732. doi: 10.3389/fphar.2025.1480732. eCollection 2025. Front Pharmacol. 2025. PMID: 40170735 Free PMC article.
-
Enhancing oocyte in vitro maturation and quality by melatonin/bilirubin cationic nanoparticles: A promising strategy for assisted reproduction techniques.Int J Pharm X. 2024 Jul 2;8:100268. doi: 10.1016/j.ijpx.2024.100268. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39070171 Free PMC article.
References
-
- Ali Shokuhi, Rad Sadegh, Mehdi, Society, A. J. C. a. p. t. o. j. o. t. K. P Potential of metal–fullerene hybrids as strong nanocarriers for cytosine and guanine nucleobases: A detailed DFT study. 2018;18:133–140.
-
- Banerjee P., Geng T., Mahanty A., Li T., Zong L., Wang B. Integrating the drug, disulfiram into the vitamin E-TPGS-modified PEGylated nanostructured lipid carriers to synergize its repurposing for anti-cancer therapy of solid tumors. Int. J. Pharm. 2019;557:374–389. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

