Chitin-glucan improves important pathophysiological features of irritable bowel syndrome
- PMID: 38690023
- PMCID: PMC11056916
- DOI: 10.3748/wjg.v30.i16.2258
Chitin-glucan improves important pathophysiological features of irritable bowel syndrome
Abstract
Background: Irritable bowel syndrome (IBS) is one of the most frequent and debilitating conditions leading to gastroenterological referrals. However, recommended treatments remain limited, yielding only limited therapeutic gains. Chitin-glucan (CG) is a novel dietary prebiotic classically used in humans at a dosage of 1.5-3.0 g/d and is considered a safe food ingredient by the European Food Safety Authority. To provide an alternative approach to managing patients with IBS, we performed preclinical molecular, cellular, and animal studies to evaluate the role of chitin-glucan in the main pathophysiological mechanisms involved in IBS.
Aim: To evaluate the roles of CG in visceral analgesia, intestinal inflammation, barrier function, and to develop computational molecular models.
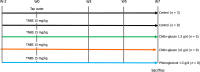
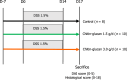
Methods: Visceral pain was recorded through colorectal distension (CRD) in a model of long-lasting colon hypersensitivity induced by an intra-rectal administration of TNBS [15 milligrams (mg)/kilogram (kg)] in 33 Sprague-Dawley rats. Intracolonic pressure was regularly assessed during the 9 wk-experiment (weeks 0, 3, 5, and 7) in animals receiving CG (n = 14) at a human equivalent dose (HED) of 1.5 g/d or 3.0 g/d and compared to negative control (tap water, n = 11) and positive control (phloroglucinol at 1.5 g/d HED, n = 8) groups. The anti-inflammatory effect of CG was evaluated using clinical and histological scores in 30 C57bl6 male mice with colitis induced by dextran sodium sulfate (DSS) administered in their drinking water during 14 d. HT-29 cells under basal conditions and after stimulation with lipopolysaccharide (LPS) were treated with CG to evaluate changes in pathways related to analgesia (µ-opioid receptor (MOR), cannabinoid receptor 2 (CB2), peroxisome proliferator-activated receptor alpha, inflammation [interleukin (IL)-10, IL-1b, and IL-8] and barrier function [mucin 2-5AC, claudin-2, zonula occludens (ZO)-1, ZO-2] using the real-time PCR method. Molecular modelling of CG, LPS, lipoteichoic acid (LTA), and phospholipomannan (PLM) was developed, and the ability of CG to chelate microbial pathogenic lipids was evaluated by docking and molecular dynamics simulations. Data were expressed as the mean ± SEM.
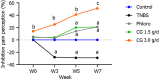
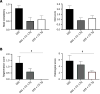
Results: Daily CG orally-administered to rats or mice was well tolerated without including diarrhea, visceral hypersensitivity, or inflammation, as evaluated at histological and molecular levels. In a model of CRD, CG at a dosage of 3 g/d HED significantly decreased visceral pain perception by 14% after 2 wk of administration (P < 0.01) and reduced inflammation intensity by 50%, resulting in complete regeneration of the colonic mucosa in mice with DSS-induced colitis. To better reproduce the characteristics of visceral pain in patients with IBS, we then measured the therapeutic impact of CG in rats with TNBS-induced inflammation to long-lasting visceral hypersensitivity. CG at a dosage of 1.5 g/d HED decreased visceral pain perception by 20% five weeks after colitis induction (P < 0.01). When the CG dosage was increased to 3.0 g/d HED, this analgesic effect surpassed that of the spasmolytic agent phloroglucinol, manifesting more rapidly within 3 wk and leading to a 50% inhibition of pain perception (P < 0.0001). The underlying molecular mechanisms contributing to these analgesic and anti-inflammatory effects of CG involved, at least in part, a significant induction of MOR, CB2 receptor, and IL-10, as well as a significant decrease in pro-inflammatory cytokines IL-1b and IL-8. CG also significantly upregulated barrier-related genes including muc5AC, claudin-2, and ZO-2. Molecular modelling of CG revealed a new property of the molecule as a chelator of microbial pathogenic lipids, sequestering gram-negative LPS and gram-positive LTA bacterial toxins, as well as PLM in fungi at the lowesr energy conformations.
Conclusion: CG decreased visceral perception and intestinal inflammation through master gene regulation and direct binding of microbial products, suggesting that CG may constitute a new therapeutic strategy for patients with IBS or IBS-like symptoms.
Keywords: Abdominal pain; Chitin-glucan; Inflammation; Intestinal barrier; Irritable bowel syndrome; Microbial cell walls chelation; Molecular modelling.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Desreumaux reports personal fees from Abbvie, personal fees from Abbott, personal fees from Amgen, personal fees from Biocodex, personal fees from Biofortis, personal fees from Biogen, personal fees from Biokuris, personal fees from Dr Falk, personal fees from Ferring, personal fees from Galapagos, personal fees from Fresenius, personal fees from Janssen, personal fees from Intestinal Biotech Development, personal fees from Kitozyme, personal fees from Lesaffre, personal fees from MSD, personal fees from Norgine, personal fees from Pfizer, personal fees from Sandoz, personal fees from Shire, personal fees from Takeda, personal fees from Tillotts, and personal fees from UCB outside the submitted work; Dr. Desreumaux has issued a patent (WO2009103884) issued; Christel Rousseaux is Chief Executive Officer at Intestinal Biotech Development; Veronique Maquet is a Product Development Manager at Kitozyme; Salvatore Modica is Chief Operating Officer at Biokuris, a spin-off company of Kitozyme; The other authors have nothing to disclose.
Figures
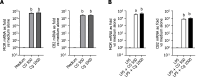
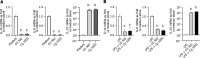
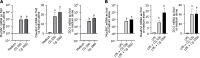
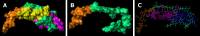

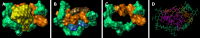
References
-
- Camilleri M, Choi MG. Review article: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:3–15. - PubMed
-
- Fortea J, Prior M. Irritable bowel syndrome with constipation: a European-focused systematic literature review of disease burden. J Med Econ. 2013;16:329–341. - PubMed
-
- Sethi S, Wadhwa V, LeClair J, Mikami S, Park R, Jones M, Sethi N, Brown A, Lembo A. In-patient discharge rates for the irritable bowel syndrome - an analysis of national trends in the United States from 1997 to 2010. Aliment Pharmacol Ther. 2013;38:1338–1346. - PubMed
-
- Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17–44. - PubMed
-
- Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, Agrawal A, Aziz I, Farmer AD, Eugenicos MP, Moss-Morris R, Yiannakou Y, Ford AC. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214–1240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

