Delineating postinfarct ventricular tachycardia substrate with dynamic voltage mapping in areas of omnipolar vector disarray
- PMID: 38690145
- PMCID: PMC11056467
- DOI: 10.1016/j.hroo.2024.02.006
Delineating postinfarct ventricular tachycardia substrate with dynamic voltage mapping in areas of omnipolar vector disarray
Abstract
Background: Defining postinfarct ventricular arrhythmic substrate is challenging with voltage mapping alone, though it may be improved in combination with an activation map. Omnipolar technology on the EnSite X system displays activation as vectors that can be superimposed onto a voltage map.
Objective: The study sought to optimize voltage map settings during ventricular tachycardia (VT) ablation, adjusting them dynamically using omnipolar vectors.
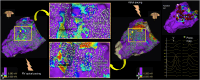
Methods: Consecutive patients undergoing substrate mapping were retrospectively studied. We categorized omnipolar vectors as uniform when pointing in one direction, or in disarray when pointing in multiple directions. We superimposed vectors onto voltage maps colored purple in tissue >1.5 mV, and the voltage settings were adjusted so that uniform vectors appeared within purple voltages, a process termed dynamic voltage mapping (DVM). Vectors in disarray appeared within red-blue lower voltages.
Results: A total of 17 substrate maps were studied in 14 patients (mean age 63 ± 13 years; mean left ventricular ejection fraction 35 ± 6%, median 4 [interquartile range 2-8.5] recent VT episodes). The DVM mean voltage threshold that differentiated tissue supporting uniform vectors from disarray was 0.27 mV, ranging between patients from 0.18 to 0.50 mV, with good interobserver agreement (median difference: 0.00 mV). We found that VT isthmus components, as well as sites of latest activation, isochronal crowding, and excellent pace maps colocated with tissue along the DVM border zone surrounding areas of disarray.
Conclusion: DVM, guided by areas of omnipolar vector disarray, allows for individualized postinfarct ventricular substrate characterization. Tissue bordering areas of disarray may harbor greater arrhythmogenic potential.
Keywords: 3D mapping; Ablation; Activation vector; Omnipolar electrograms; Postinfarct scar; Ventricular tachycardia.
© 2024 Heart Rhythm Society. Published by Elsevier Inc.
Conflict of interest statement
Nicole Worthington and Calum Phenton are employees of Abbott Medical and offered manuscript quality assurance. All other authors declare no conflicts of interest relevant to this study.
Figures




References
-
- Ciaccio E.J., Anter E., Coromilas J., et al. Structure and function of the ventricular tachycardia isthmus. Heart Rhythm. 2022;19:137–153. - PubMed
-
- Cassidy D.M., Vassallo J.A., Miller J.M., et al. Endocardial catheter mapping in patients in sinus rhythm: relationship to underlying heart disease and ventricular arrhythmias. Circulation. 1986;73:645–652. - PubMed
-
- Marchlinski F.E., Callans D.J., Gottlieb C.D., Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. - PubMed
-
- Josephson M.E., Anter E. Substrate mapping for ventricular tachycardia. JACC Clin Electrophysiol. 2015;1:341–352. - PubMed
-
- Soejima K., Stevenson W.G., Maisel W.H., et al. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106:1678–1683. - PubMed
LinkOut - more resources
Full Text Sources

