Totally implantable venous ports in infants and children: a single-center retrospective study of indications and safety
- PMID: 38690159
- PMCID: PMC11058838
- DOI: 10.3389/fonc.2024.1351630
Totally implantable venous ports in infants and children: a single-center retrospective study of indications and safety
Abstract
Introduction: Totally Implantable Venous Access Devices (TIVADs) contribute significantly to the treatment progress and comfort of patients requiring long-term therapy. However, the procedure for implanting TIVADs, as well as its very presence, may be associated with complications.
Aim: This study evaluates the indications, safety, and complication rates of venous port implantations in pediatric patients. It also explores factors influencing the occurrence of early and late complications post-implantation.
Materials and methods: The study included 383 pediatric patients treated at the Department of Pediatric Surgery, Traumatology, and Urology in Poznan between 2013 and 2020 who underwent 474 implantations of intravenous ports. Venous access was achieved using the Seldinger technique. Statistical analysis was performed using Statistica 13 with TIBCO and PQStat 1.8.2.156 with PQStat.
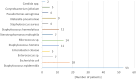
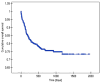
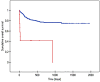
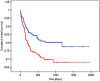
Results: Venous ports were used in 345 oncology patients requiring chemotherapy (90% of the total group) and in 38 children (10%) with non-oncology indications. There were 36 early complications (7.6%) and 18 late complications (3.8%), excluding infectious complications. The most common early, non-infectious complications included pneumothorax (15 patients; 3%) and port pocket hematoma (12 patients; 2.5%). The most common late, non-infectious complications observed were venous catheter obstruction (8 children; 1.7%) and port system leakage (5 children; 1%). Infectious complications occurred in 129 cases (27.2%). Children with a diagnosis of non-Hodgkin's lymphoma, acute myeloid leukemia, and acute lymphoblastic leukemia had a significantly higher incidence of port infections. Venous ports equipped with a polyurethane catheter, compared to systems with a silicone catheter, functioned significantly shorter.
Conclusions: The Seldinger method of port implantation is quick, minimally invasive, and safe. The type of port, including the material of the port's venous catheter, and the underlying disease have an impact on the durability of implantable intravenous systems. The experience of the surgeon is related to the frequency of complications associated with the procedure.
Keywords: Seldinger method; child; complications; infections; long-term access; venous port.
Copyright © 2024 Sosnowska-Sienkiewicz, Moryciński, Januszkiewicz-Lewandowska, Michalik, Madziar, Kukfisz, Zielińska and Mańkowski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Dogar SA, Khan MA. Implantable port devices in pediatric oncology patients: a clinical experience from a tertiary care hospital. J Pak Med Assoc. (2013) 63:1248–51. - PubMed
LinkOut - more resources
Full Text Sources

