Three-year follow-up analysis of phase 1/2 study on tirabrutinib in patients with relapsed or refractory primary central nervous system lymphoma
- PMID: 38690230
- PMCID: PMC11059299
- DOI: 10.1093/noajnl/vdae037
Three-year follow-up analysis of phase 1/2 study on tirabrutinib in patients with relapsed or refractory primary central nervous system lymphoma
Abstract
Background: The ONO-4059-02 phase 1/2 study showed favorable efficacy and acceptable safety profile of tirabrutinib, a second-generation Bruton's tyrosine kinase inhibitor, for relapsed/refractory primary central nervous system lymphoma (PCNSL). Here, we report the long-term efficacy and safety after a 3-year follow-up.
Methods: Eligible patients were aged ≥ 20 years with histologically diagnosed PCNSL and KPS of ≥ 70. Patients received oral tirabrutinib once daily at 320 or 480 mg, or 480 mg under fasted conditions.
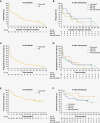
Results: Between October 19, 2017, and June 13, 2019, 44 patients were enrolled: 33 and 9 had relapsed and refractory, respectively. The 320, 480, and 480 mg fasted groups included 20, 7, and 17 patients, respectively. The median follow-up was 37.1 months. The overall response rate was 63.6% (95% CI: 47.8-77.6) with complete response (CR), unconfirmed CR, and partial response in 9, 7, and 12 patients, respectively. The median duration of response (DOR) was 9.2 months, with a DOR rate of 19.8%; the median progression-free survival (PFS) and median overall survival (OS) were 2.9 months and not reached, respectively, with PFS and OS rates of 13.9% and 56.7%, respectively. Adverse events occurred in 38 patients (86.4%): grade ≥ 3 in 23 (52.3%) including 1 patient with grade 5 events. KPS and quality of life (QoL) scores were well maintained among patients receiving long-term treatment.
Conclusions: The results demonstrated the long-term clinical benefit of tirabrutinib, with deep and durable response in a subset of patients and acceptable safety profile, while KPS and QoL scores were maintained.
Keywords: BTKi; Bruton’s tyrosine kinase inhibitor; ONO-4059; PCNSL; primary central nervous system lymphoma; tirabrutinib.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
All authors received support for developing the manuscript, which includes funding, medical writing, and article processing charge. HY reports honoraria from Ono, Chugai, Fujifilm, and Novocure global. YN reports grants from Chugai, Sumitomo Pharma, Eisai, Otsuka, SBI, AbbVie, Daiichi Sankyo, Stella Pharma, and Meiji Seika; honoraria from Chugai, Sumitomo Pharma, Eisai, Otsuka, SBI, AbbVie, Daiichi Sankyo, Stella Pharma, and Meiji Seika. MN reports grants from Chugai, MSD, Nippon Kayaku, Bristol Myers Squibb, Pfizer, Takeda, Shionogi, Kyowa Kirin, Teijin Pharma, Asahi Kasei Medical, HOYA Technosurgical, AbbVie, Eisai, Daiichi Sankyo, Otsuka, Astellas, Tsumura, Sanofi, Mitsubishi Tanabe Pharma, Sanei, CSF Behring, and Ono; consulting fees from Ono, Nippon Shinyaku, and Novocure; honoraria from Chugai, MSD, Nippon Kayaku, UCB Japan, Sumitomo Pharma, Ono, Ohara, AbbVie, Eisai, Daiichi Sankyo, Novocure, Bristol Myers Squibb, and Kyowa Kirin; support for attending meetings and/or travel from Ono, Eisai, Denka, Kyowa Kirin, and Nippon Kayaku; Participation on a Data Safety Monitoring Board or Advisory Board in Novocure; and medical writing support from Ono and Chugai. KM reports grants from Chugai, Eisai, Gunze Medical, Otsuka, Nihon Medi-Physics, Gunze, Stryker Japan, Kyowa Kirin, MSD, Teijin Pharma, AbbVie, Daiichi Sankyo, Novocure, HOYA Technosurgical, Ohara, and CSL Behring; and honoraria from Ono and Chugai. YA reports grants from Philips, Otsuka, Chugai, Nihon Medi-Physics, Daiichi Sankyo, Stryker, Eisai, Japan Blood Products Organization, Ono, Taiho, Sumitomo Pharma, Astellas, Incyte Biosciences, and Servier; and honoraria for lectures from Nippon Kayaku, Novocure, UCB Japan, Ono, Brainlab, Merck, Chugai, Eisai, Daiichi Sankyo, Carl Zeiss, and Nihon Medi-Physics. KA reports honoraria and support for attending meetings and/or travel from Ono. NF reports grants from Ono, Bayer, Chugai, Celgene, and Genmab and Incyte; honoraria from AstraZeneca, Bristol Myers Squibb, Chugai, CSL Behring, Sumitomo Pharma, Eisai, Janssen, Kyowa Kirin, Nippon Shinyaku, Novartis, Ono, Otsuka, Sanofi, SymBio, Takeda, and Zenyaku; and participation on a data safety monitoring board in Huya Japan and an advisory board in AstraZeneca, AbbVie, Eli Lilly, Genmab, and Novartis. KS reports honoraria from Daiichi Sankyo, Eisai, Meiji Seika Pharma, Bristol Myers Squibb, Novartis, Ono, and Nobel Pharma. AA is employed in Ono and holds stocks of Ono. RN reports grants from MSD, Eisai, AbbVie, and Chugai; and consulting fee from Novocure; honoraria from AbbVie, Chugai, Daiichi Sankyo, Eisai, Novocure, and Ono.
Figures

References
-
- Ferreri AJM. How I treat primary CNS lymphoma. Blood. 2011;118(3):510–522. - PubMed
-
- Camilleri-Broët S, Martin A, Moreau A, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: Pathologic findings and clinical correlations. Am J Clin Pathol. 1998;110(5):607–612. - PubMed
-
- Lin CH, Kuo KT, Chuang SS, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12(4):1152–1156. - PubMed
-
- Alizadeh AA, Elsen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. - PubMed
LinkOut - more resources
Full Text Sources
