The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps
- PMID: 38690264
- PMCID: PMC11059040
- DOI: 10.3389/fimmu.2024.1356298
The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps
Abstract
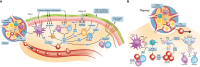
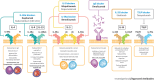
Chronic rhinosinusitis with nasal polyps (CRSwNP) is predominantly a type 2 inflammatory disease associated with type 2 (T2) cell responses and epithelial barrier, mucociliary, and olfactory dysfunction. The inflammatory cytokines interleukin (IL)-4, IL-13, and IL-5 are key mediators driving and perpetuating type 2 inflammation. The inflammatory responses driven by these cytokines include the recruitment and activation of eosinophils, basophils, mast cells, goblet cells, M2 macrophages, and B cells. The activation of these immune cells results in a range of pathologic effects including immunoglobulin E production, an increase in the number of smooth muscle cells within the nasal mucosa and a reduction in their contractility, increased deposition of fibrinogen, mucus hyperproduction, and local edema. The cytokine-driven structural changes include nasal polyp formation and nasal epithelial tissue remodeling, which perpetuate barrier dysfunction. Type 2 inflammation may also alter the availability or function of olfactory sensory neurons contributing to loss of sense of smell. Targeting these key cytokine pathways has emerged as an effective approach for the treatment of type 2 inflammatory airway diseases, and a number of biologic agents are now available or in development for CRSwNP. In this review, we provide an overview of the inflammatory pathways involved in CRSwNP and describe how targeting key drivers of type 2 inflammation is an effective therapeutic option for patients.
Keywords: CRSwNP; biologic; chronic rhinosinusitis with nasal polyps; cytokine; interleukin-13; interleukin-4; interleukin-4 receptor; type 2 inflammation.
Copyright © 2024 Bachert, Hicks, Gane, Peters, Gevaert, Nash, Horowitz, Sacks and Jacob-Nara.
Conflict of interest statement
CB is an advisory board member for ALK, ASIT Biotech,AstraZeneca, GlaxoSmithKline, Intrexon Actobiotics, Novartis, Sanofi, and Stallergenes Greer. AH and JJ-N are employees and may hold stock and/or stock options in Sanofi. SG reports advisory board fees from Sanofi and GlaxoSmithKline. AP reports advisory board fees and research support from AstraZeneca, Regeneron Pharmaceuticals, Inc., and Sanofi, and consulting fees and research support from Optinose. PG reports clinical trial funding from and is an advisory board member of 3NT, Argenx, Genentech, Novartis, Regeneron Pharmaceuticals Inc., Roche, Sanofi, and Stallergenes Greer. SN, JH, and HS are employees of and may hold stock and/or stock options in Regeneron Pharmaceuticals Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

