Asia-Pacific consensus on long-term and sequential therapy for osteoporosis
- PMID: 38690538
- PMCID: PMC11056428
- DOI: 10.1016/j.afos.2024.02.001
Asia-Pacific consensus on long-term and sequential therapy for osteoporosis
Abstract
Objectives: This study aimed to present the Asia-Pacific consensus on long-term and sequential therapy for osteoporosis, offering evidence-based recommendations for the effective management of this chronic condition. The primary focus is on achieving optimal fracture prevention through a comprehensive, individualized approach.
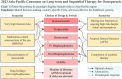
Methods: A panel of experts convened to develop consensus statements by synthesizing the current literature and leveraging clinical expertise. The review encompassed long-term anti-osteoporosis medication goals, first-line treatments for individuals at very high fracture risk, and the strategic integration of anabolic and antiresorptive agents in sequential therapy approaches.
Results: The panelists reached a consensus on 12 statements. Key recommendations included advocating for anabolic agents as the first-line treatment for individuals at very high fracture risk and transitioning to antiresorptive agents following the completion of anabolic therapy. Anabolic therapy remains an option for individuals experiencing new fractures or persistent high fracture risk despite antiresorptive treatment. In cases of inadequate response, the consensus recommended considering a switch to more potent medications. The consensus also addressed the management of medication-related complications, proposing alternatives instead of discontinuation of treatment.
Conclusions: This consensus provides a comprehensive, cost-effective strategy for fracture prevention with an emphasis on shared decision-making and the incorporation of country-specific case management systems, such as fracture liaison services. It serves as a valuable guide for healthcare professionals in the Asia-Pacific region, contributing to the ongoing evolution of osteoporosis management.
Keywords: Anti-osteoporosis medication; Asia–Pacific; Consensus; Fracture prevention; Sequential therapy.
© 2024 The Korean Society of Osteoporosis. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors disclosed the following conflicts of interest.1.Ta-Wei Tai received honoraria for lectures, meetings, and/or travel from Amgen and Alvogen/Lotus.2.Swan Sim Yeap has received honoraria for lectures from Amgen.3.Natthinee Charatcharoenwitthaya received honoraria for lectures, meetings, and/or travel from Amgen, Alvogen, and Zuellig Pharma.4.Akira Taguchi has received lecture fees from Asahi Kasei Pharma Corp., Daiichi Sankyo Co. Ltd, Chugai Pharmaceutical Co. Ltd, and Teijin Pharma Ltd.5.Peter R Ebeling has received research funding from Amgen, Alexion and Sanofi, and honoraria from Amgen, Alexion and Kyowa Kirin.6.Fernando Marin has received honoraria for lectures from DKSH and Zuellig Pharma. He is a former employee of Eli Lilly and Company.7.Yoon-Sok Chung has received research funding from Samsung Bioepis and honoraria from Amgen, Alvogen, Celltrion, Daewoong, Hanlim, and Yuyu.8.Ian R Reid has received speaking fees from Amgen and Medison Pharma.9.Manju Chandran has received honoraria and travel sponsorships from Amgen, DKSH, and Kyowa Kirin.10.E. Michael Lewiecki - Amgen: investigator, consultant, speaker; Radius: investigator, consultant; Kyowa Kirin: consultant, speaker; Ultragenyx: investigator; Angitia: consultant; Ascendis: consultant.11.Fen Lee Hew has received honoraria from Amgen and DKSH.12.Tuan Van Nguyen has received a global competitive grant from Amgen and honoraria from Amgen, DKSH, and Bridge Health Care, for giving lectures and travelling to meetings.13.Chung-Hwan Chen received honoraria for lectures, attending meetings, and/or travel from Amgen, and Alvogen/Lotus.14.Chih-Hsing Wu received honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier laboratories, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus.15.The other authors reported that they have nothing to declare for potential conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources