A Laing distal myopathy-associated proline substitution in the β-myosin rod perturbs myosin cross-bridging activity
- PMID: 38690726
- PMCID: PMC11060730
- DOI: 10.1172/JCI172599
A Laing distal myopathy-associated proline substitution in the β-myosin rod perturbs myosin cross-bridging activity
Abstract
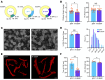
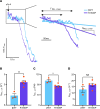
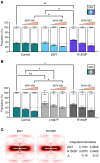
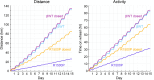
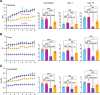
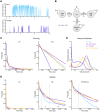
Proline substitutions within the coiled-coil rod region of the β-myosin gene (MYH7) are the predominant mutations causing Laing distal myopathy (MPD1), an autosomal dominant disorder characterized by progressive weakness of distal/proximal muscles. We report that the MDP1 mutation R1500P, studied in what we believe to be the first mouse model for the disease, adversely affected myosin motor activity despite being in the structural rod domain that directs thick filament assembly. Contractility experiments carried out on isolated mutant muscles, myofibrils, and myofibers identified muscle fatigue and weakness phenotypes, an increased rate of actin-myosin detachment, and a conformational shift of the myosin heads toward the more reactive disordered relaxed (DRX) state, causing hypercontractility and greater ATP consumption. Similarly, molecular analysis of muscle biopsies from patients with MPD1 revealed a significant increase in sarcomeric DRX content, as observed in a subset of myosin motor domain mutations causing hypertrophic cardiomyopathy. Finally, oral administration of MYK-581, a small molecule that decreases the population of heads in the DRX configuration, significantly improved the limited running capacity of the R1500P-transgenic mice and corrected the increased DRX state of the myofibrils from patients. These studies provide evidence of the molecular pathogenesis of proline rod mutations and lay the groundwork for the therapeutic advancement of myosin modulators.
Keywords: Molecular pathology; Mouse models; Muscle; Muscle biology.
Figures

Comment in
- A therapeutic leap: How myosin inhibitors moved from cardiac interventions to skeletal muscle myopathy solutions doi: 10.1172/JCI179958
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

