BAP1 is required prenatally for differentiation and maintenance of postnatal murine enteric nervous system
- PMID: 38690732
- PMCID: PMC11060734
- DOI: 10.1172/JCI177771
BAP1 is required prenatally for differentiation and maintenance of postnatal murine enteric nervous system
Abstract
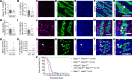
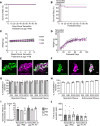
Epigenetic regulatory mechanisms are underappreciated, yet are critical for enteric nervous system (ENS) development and maintenance. We discovered that fetal loss of the epigenetic regulator Bap1 in the ENS lineage caused severe postnatal bowel dysfunction and early death in Tyrosinase-Cre Bap1fl/fl mice. Bap1-depleted ENS appeared normal in neonates; however, by P15, Bap1-deficient enteric neurons were largely absent from the small and large intestine of Tyrosinase-Cre Bap1fl/fl mice. Bowel motility became markedly abnormal with disproportionate loss of cholinergic neurons. Single-cell RNA sequencing at P5 showed that fetal Bap1 loss in Tyrosinase-Cre Bap1fl/fl mice markedly altered the composition and relative proportions of enteric neuron subtypes. In contrast, postnatal deletion of Bap1 did not cause enteric neuron loss or impaired bowel motility. These findings suggest that BAP1 is critical for postnatal enteric neuron differentiation and for early enteric neuron survival, a finding that may be relevant to the recently described human BAP1-associated neurodevelopmental disorder.
Keywords: Development; Gastroenterology; Neurodegeneration.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

