Lipidation alters the phase-separation of resilin-like polypeptides
- PMID: 38690757
- PMCID: PMC11095499
- DOI: 10.1039/d4sm00358f
Lipidation alters the phase-separation of resilin-like polypeptides
Abstract
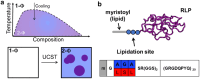
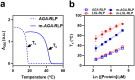
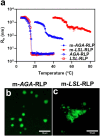
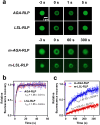
Biology exploits biomacromolecular phase separation to form condensates, known as membraneless organelles. Despite significant advancements in deciphering sequence determinants for phase separation, modulating these features in vivo remains challenging. A promising approach inspired by biology is to use post-translational modifications (PTMs)-to modulate the amino acid physicochemistry instead of altering protein sequences-to control the formation and characteristics of condensates. However, despite the identification of more than 300 types of PTMs, the detailed understanding of how they influence the formation and material properties of protein condensates remains incomplete. In this study, we investigated how modification with myristoyl lipid alters the formation and characteristics of the resilin-like polypeptide (RLP) condensates, a prototypical disordered protein with upper critical solution temperature (UCST) phase behaviour. Using turbidimetry, dynamic light scattering, confocal and electron microscopy, we demonstrated that lipidation-in synergy with the sequence of the lipidation site-significantly influences RLPs' thermodynamic propensity for phase separation and their condensate properties. Molecular simulations suggested these effects result from an expanded hydrophobic region created by the interaction between the lipid and lipidation site rather than changes in peptide rigidity. These findings emphasize the role of "sequence context" in modifying the properties of PTMs, suggesting that variations in lipidation sequences could be strategically used to fine-tune the effect of these motifs. Our study advances understanding of lipidation's impact on UCST phase behaviour, relevant to proteins critical in biological processes and diseases, and opens avenues for designing lipidated resilins for biomedical applications like heat-mediated drug elution.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Temperature-triggered phase separation of a hydrophilic resilin-like polypeptide.Macromol Rapid Commun. 2015 Jan;36(1):90-5. doi: 10.1002/marc.201400521. Epub 2014 Nov 26. Macromol Rapid Commun. 2015. PMID: 25424611 Free PMC article.
-
Sequence Context and Complex Hofmeister Salt Interactions Dictate Phase Separation Propensity of Resilin-like Polypeptides.Biomacromolecules. 2022 Dec 12;23(12):5225-5238. doi: 10.1021/acs.biomac.2c01027. Epub 2022 Nov 15. Biomacromolecules. 2022. PMID: 36378745
-
Alteration of Microstructure in Biopolymeric Hydrogels via Compositional Modification of Resilin-Like Polypeptides.ACS Biomater Sci Eng. 2021 Sep 13;7(9):4244-4257. doi: 10.1021/acsbiomaterials.0c01543. Epub 2021 Jan 19. ACS Biomater Sci Eng. 2021. PMID: 33464811
-
Resilin-mimetics as a smart biomaterial platform for biomedical applications.Nat Commun. 2021 Jan 8;12(1):149. doi: 10.1038/s41467-020-20375-x. Nat Commun. 2021. PMID: 33420053 Free PMC article. Review.
-
Theories for Sequence-Dependent Phase Behaviors of Biomolecular Condensates.Biochemistry. 2018 May 1;57(17):2499-2508. doi: 10.1021/acs.biochem.8b00058. Epub 2018 Mar 13. Biochemistry. 2018. PMID: 29509422 Review.
Cited by
-
Toward understanding biomolecular materials comprising intrinsically disordered proteins via simulation and experiment.Mol Syst Des Eng. 2025 Apr 25;10(7):502-518. doi: 10.1039/d4me00197d. eCollection 2025 Jun 30. Mol Syst Des Eng. 2025. PMID: 40386766 Free PMC article. Review.
-
Scalable One-Pot Production of Geranylgeranylated Proteins in Engineered Prokaryotes.Bioconjug Chem. 2025 Mar 19;36(3):415-423. doi: 10.1021/acs.bioconjchem.4c00493. Epub 2025 Mar 3. Bioconjug Chem. 2025. PMID: 40029010 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

