Sleep-wake behavior, perceived fatigability, and cognitive reserve in older adults
- PMID: 38690777
- PMCID: PMC11180948
- DOI: 10.1002/alz.13802
Sleep-wake behavior, perceived fatigability, and cognitive reserve in older adults
Abstract
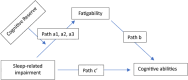
Introduction: The effects of sleep-wake behavior on perceived fatigability and cognitive abilities when performing daily activities have not been investigated across levels of cognitive reserve (CR).
Methods: CR Index Questionnaire (CRIq) data were collected and subjected to moderated mediation analysis.
Results: In amnestic mild cognitive impairment (aMCI; n = 41), CR moderated sleep-related impairments (SRIs), and fatigability at low CR (CRIq < 105.8, p = 0.004) and mean CR (CRIq = 126.9, p = 0.03) but not high CR (CRIq > 145.9, p = 0.65) levels. SRI affected cognitive abilities mediated by fatigability at low CR (p < 0.001) and mean CR (p = 0.003) levels. In healthy controls (n = 13), SRI in fatigability did not alter cognitive abilities across CR levels; controls had higher leisure scores than patients with aMCI (p = 0.003, effect size = 0.93).
Discussion: SRI can amplify impaired cognitive abilities through exacerbation of fatigability in patients with aMCI with below-mean CR. Therefore, improving sleep-wake regulation and leisure activities may protect against fatigability and cognitive decline.
Highlights: Clinical fatigue and fatigability cannot be alleviated by rest. Clinical fatigability disrupts daily activities during preclinical Alzheimer's. High cognitive reserve mitigates sleep-wake disturbance effects. High cognitive reserve attenuates clinical fatigability effects on daily functioning. Untreated obstructive sleep apnea potentiates Alzheimer's pathology in the brain.
Keywords: Alzheimer's disease; cognitive ability; cognitive reserve; fatigue; leisure activities; older adults; sleep; sleep fragmentation; sleep–wake behavior; sleep–wake dysregulation; sleep–wake misalignment.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Kerner receives support from Columbia University's Clinical and Translational Research (CTSA) and NIH/NIA. Drs. Goldberg, Qin, Andrews, Pelton, and Devanand receive support from the NIH/NIA. All authors have no conflict of interest to declare. Author disclosures are available in the supporting information.
Figures


References
-
- Naismith SL, Hickie IB, Terpening Z, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857‐866. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

