An updated list of eumycetoma causative agents and their differences in grain formation and treatment response
- PMID: 38690871
- PMCID: PMC11237709
- DOI: 10.1128/cmr.00034-23
An updated list of eumycetoma causative agents and their differences in grain formation and treatment response
Abstract
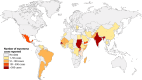
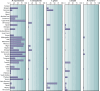
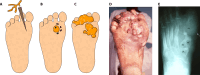
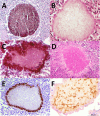
SUMMARYIn 2023, the World Health Organization designated eumycetoma causative agents as high-priority pathogens on its list of fungal priority pathogens. Despite this recognition, a comprehensive understanding of these causative agents is lacking, and potential variations in clinical manifestations or therapeutic responses remain unclear. In this review, 12,379 eumycetoma cases were reviewed. In total, 69 different fungal species were identified as causative agents. However, some were only identified once, and there was no supporting evidence that they were indeed present in the grain. Madurella mycetomatis was by far the most commonly reported fungal causative agent. In most studies, identification of the fungus at the species level was based on culture or histology, which was prone to misidentifications. The newly used molecular identification tools identified new causative agents. Clinically, no differences were reported in the appearance of the lesion, but variations in mycetoma grain formation and antifungal susceptibility were observed. Although attempts were made to explore the differences in clinical outcomes based on antifungal susceptibility, the lack of large clinical trials and the inclusion of surgery as standard treatment posed challenges in drawing definitive conclusions. Limited case series suggested that eumycetoma cases caused by Fusarium species were less responsive to treatment than those caused by Madurella mycetomatis. However, further research is imperative for a comprehensive understanding.
Keywords: biofilm; diagnosis; grain; itraconazole; molecular diagnostics; mycetoma; neglected tropical disease; susceptibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
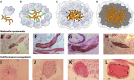
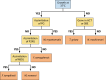
References
-
- WHO . 2016. WHA69.21: assessing the burden of mycetoma. WHO, Geneva.
-
- WHO . 2023. WHO fungal priority pathogens list to guide research, development and public health action
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

