Validation of a qualitative real-time PCR assay for the detection of Candida auris in hospital inpatient screening
- PMID: 38690882
- PMCID: PMC11237412
- DOI: 10.1128/jcm.00158-24
Validation of a qualitative real-time PCR assay for the detection of Candida auris in hospital inpatient screening
Abstract
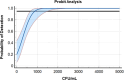
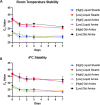
Candida auris is a multidrug-resistant opportunistic fungal pathogen capable of causing serious infections and healthcare-associated outbreaks. Screening for colonization with C. auris has become routine and is recommended in many hospitals and healthcare facilities as an infection control and prevention strategy. Subsequently, and since there are currently no FDA-approved tests for this purpose, clinical microbiology laboratories have become responsible for developing protocols to detect C. auris using axial and inguinal screening swabs. In a College of American Pathologists-accredited large academic healthcare center setting, we implemented a laboratory-developed nucleic-acid amplification test for the detection of C. auris DNA. Our test validation evaluated the performance of the DiaSorin C. auris primer set used in a real-time qualitative PCR assay on the LIAISON MDX thermocycler with the Simplexa Universal Disc. The assay was highly sensitive and specific, with a limit of detection of 1-2 CFU/reaction, with no observed cross-reactivity with other Candida spp., bacterial skin commensal organisms or commonly encountered viruses. When run in parallel with a culture-based detection method, the PCR assay was 100% sensitive and specific. The assay was precise, with low variability between replicates within and between runs. Lastly, pre-analytical factors, including swab storage time, temperature, and transport media, were assessed and found to have no significant effect on the detection of C. auris at variable concentrations. Taken together, this study expands the available options for nucleic acid detection of C. auris and characterizes pre-analytical factors for implementation in both high- and low-volume laboratory settings.
Importance: This study overviews the validation and implementation of a molecular screening tool for the detection of Candida auris in a College of American Pathologist-accredited clinical laboratory. This molecular laboratory-developed test is both highly sensitive and specific and has significant health-system cost-savings associated with significantly reduced turn-around-time compared to traditional standard-of-care culture-based work up. This method and workflow is of interest to support clinical microbiology diagnostics and to help aid in hospital inpatient, and infection prevention control screening.
Keywords: Candida auris; hospital infections; infectious disease; molecular methods; mycology; public health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

