Calcium modulation of bacterial wilt disease on potato
- PMID: 38690890
- PMCID: PMC11107177
- DOI: 10.1128/aem.00242-24
Calcium modulation of bacterial wilt disease on potato
Abstract
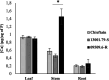
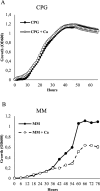
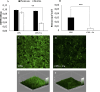
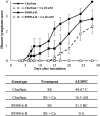
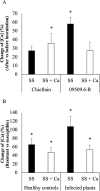
Ralstonia solanacearum species complex (RSSC) is a phytopathogenic bacterial group that causes bacterial wilt in several crops, being potato (Solanum tuberosum) one of the most important hosts. The relationship between the potato plant ionome (mineral and trace elements composition) and the resistance levels to this pathogen has not been addressed until now. Mineral content of xylem sap, roots, stems and leaves of potato genotypes with different levels of resistance to bacterial wilt was assessed in this work, revealing a positive correlation between divalent calcium (Ca) cation concentrations and genotype resistance. The aim of this study was to investigate the effect of Ca on bacterial wilt resistance, and on the growth and virulence of RSSC. Ca supplementation significantly decreased the growth rate of Ralstonia pseudosolanacearum GMI1000 in minimal medium and affected several virulence traits such as biofilm formation and twitching motility. We also incorporate for the first time the use of microfluidic chambers to follow the pathogen growth and biofilm formation in conditions mimicking the plant vascular system. By using this approach, a reduction in biofilm formation was observed when both, rich and minimal media, were supplemented with Ca. Assessment of the effect of Ca amendments on bacterial wilt progress in potato genotypes revealed a significant delay in disease progress, or a complete absence of wilting symptoms in the case of partially resistant genotypes. This work contributes to the understanding of Ca effect on virulence of this important pathogen and provides new strategies for an integrated control of bacterial wilt on potato.
Importance: Ralstonia solanacearum species complex (RSSC) includes a diverse group of bacterial strains that cause bacterial wilt. This disease is difficult to control due to pathogen aggressiveness, persistence, wide range of hosts, and wide geographic distribution in tropical, subtropical, and temperate regions. RSSC causes considerable losses depending on the pathogen strain, host, soil type, environmental conditions, and cultural practices. In potato, losses of $19 billion per year have been estimated for this pathogen worldwide. In this study, we report for the first time the mineral composition found in xylem sap and plant tissues of potato germplasm with different levels of resistance to bacterial wilt. This study underscores the crucial role of calcium (Ca) concentration in the xylem sap and stem in relation to the resistance of different genotypes. Our in vitro experiments provide evidence of Ca's inhibitory effect on the growth, biofilm formation, and twitching movement of the model RSSC strain R. pseudosolanacearum GMI1000. This study introduces a novel element, the Ca concentration, which should be included into the integrated disease control management strategies for bacterial wilt in potatoes.
Keywords: bacterial wilt; calcium; disease resistance; plant ionome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Birch PRJ, Bryan G, Fenton B, Gilroy EM, Hein I, Jones JT, Prashar A, Taylor MA, Torrance L, Toth IK. 2012. Crops that feed the world 8: potato: are the trends of increased global production sustainable? Food Sec 4:477–508. doi: 10.1007/s12571-012-0220-1 - DOI
-
- Elphinstone JG. 2005. The current bacterial wilt situation: a global overview, p 9–28. In Bact wilt dis Ralstonia solanacearum species complex.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

