Insights into the activity of cefiderocol against PER-2 producing Enterobacterales
- PMID: 38690895
- PMCID: PMC11620487
- DOI: 10.1128/aac.01720-23
Insights into the activity of cefiderocol against PER-2 producing Enterobacterales
Abstract
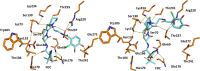
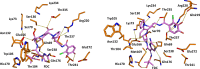
The PER-2 β-lactamase is a unique class A enzyme conferring broad spectrum cephalosporin resistance. In this study, we explored the stability of cefiderocol (FDC) against PER-2 β-lactamase to gain insights into structure activity relationships (SAR) of this synthetic siderophore-conjugated antibiotic. Herein, we show that the MICs of FDC for PER-2 producing isolates and transformants ranged between 0.125 and 64 µg/mL; diazabicyclooctanes (DBOs) reduced the MIC values. In PER-2 mutants, MIC values decreased up to 10-12 dilutions in agreement with previous observations especially in the case of Arg220 substitutions. Catalytic efficiency for PER-2 was 0.072 µM-1 s-1, comparable with PER-1 (0.046 µM-1 s-1) and NDM-1 (0.067 µM-1 s-1). In silico models revealed that FDC within the active site of PER-2 demonstrates unique interactions as a result of the inverted Ω loop fold and extension of the β3-β4 connecting loop.
Keywords: Arg220; ESBL; diazabicyclooctanes; docking; enzyme kinetics; siderophore-conjugated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

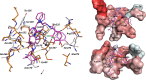
References
-
- Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi:10.1128/AAC.01405-16 - DOI - PMC - PubMed
-
- Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2018. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. doi:10.1128/AAC.01454-17 - DOI - PMC - PubMed
-
- Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, Domitrovic TN, Rudin SD, Richter SS, van Duin D, Kreiswirth BN, Greco C, Fouts DE, Bonomo RA. 2019. ARGONAUT-I: activity of cefiderocol (S-649266) a siderophore cephalosporin, against Gram-negative bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum Β-lactamases and carbapenemases. Antimicrob Agents Chemother 63:e01801-18. doi:10.1128/AAC.01801-18 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

