Molecular functions of ANKLE2 and its implications in human disease
- PMID: 38691001
- PMCID: PMC11103583
- DOI: 10.1242/dmm.050554
Molecular functions of ANKLE2 and its implications in human disease
Abstract
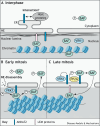
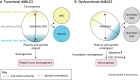
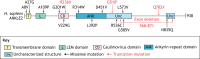
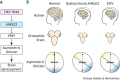
Ankyrin repeat and LEM domain-containing 2 (ANKLE2) is a scaffolding protein with established roles in cell division and development, the dysfunction of which is increasingly implicated in human disease. ANKLE2 regulates nuclear envelope disassembly at the onset of mitosis and its reassembly after chromosome segregation. ANKLE2 dysfunction is associated with abnormal nuclear morphology and cell division. It regulates the nuclear envelope by mediating protein-protein interactions with barrier to autointegration factor (BANF1; also known as BAF) and with the kinase and phosphatase that modulate the phosphorylation state of BAF. In brain development, ANKLE2 is crucial for proper asymmetric division of neural progenitor cells. In humans, pathogenic loss-of-function mutations in ANKLE2 are associated with primary congenital microcephaly, a condition in which the brain is not properly developed at birth. ANKLE2 is also linked to other disease pathologies, including congenital Zika syndrome, cancer and tauopathy. Here, we review the molecular roles of ANKLE2 and the recent literature on human diseases caused by its dysfunction.
Keywords: Cell division; Microcephaly; Neurodevelopment.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Adachi, K., Romero, T., Nielsen-Saines, K., Pone, S., Aibe, M., Barroso de Aguiar, E., Sim, M., Brasil, P., Zin, A., Tsui, I.et al. (2020). Early clinical infancy outcomes for microcephaly and/or small for gestational age Zika-exposed infants. Clin. Infect. Dis. 70, 2663-2672. 10.1093/cid/ciz704 - DOI - PMC - PubMed
-
- Apridita Sebastian, W., Shiraishi, H., Shimizu, N., Umeda, R., Lai, S., Ikeuchi, M., Morisaki, I., Yano, S., Yoshimura, A., Hanada, R.et al. (2022). Ankle2 deficiency-associated microcephaly and spermatogenesis defects in zebrafish are alleviated by heterozygous deletion of vrk1. Biochem. Biophys. Res. Commun. 624, 95-101. 10.1016/j.bbrc.2022.07.070 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

