From bristle to brain: embryonic development of topographic projections from basiconic sensilla in the antennal nervous system of the locust Schistocerca gregaria
- PMID: 38691194
- PMCID: PMC11226553
- DOI: 10.1007/s00427-024-00716-2
From bristle to brain: embryonic development of topographic projections from basiconic sensilla in the antennal nervous system of the locust Schistocerca gregaria
Abstract
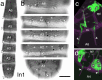
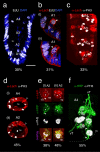
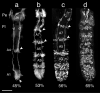
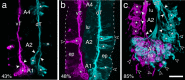
The antennal flagellum of the locust S. gregaria is an articulated structure bearing a spectrum of sensilla that responds to sensory stimuli. In this study, we focus on the basiconic-type bristles as a model for sensory system development in the antenna. At the end of embryogenesis, these bristles are found at fixed locations and then on only the most distal six articulations of the antenna. They are innervated by a dendrite from a sensory cell cluster in the underlying epithelium, with each cluster directing fused axons topographically to an antennal tract running to the brain. We employ confocal imaging and immunolabeling to (a) identify mitotically active sense organ precursors for sensory cell clusters in the most distal annuli of the early embryonic antenna; (b) observe the subsequent spatial appearance of their neuronal progeny; and (c) map the spatial and temporal organization of axon projections from such clusters into the antennal tracts. We show that early in embryogenesis, proliferative precursors are localized circumferentially within discrete epithelial domains of the flagellum. Progeny first appear distally at the antennal tip and then sequentially in a proximal direction so that sensory neuron populations are distributed in an age-dependent manner along the antenna. Autotracing reveals that axon fasciculation with a tract is also sequential and reflects the location and age of the cell cluster along the most distal annuli. Cell cluster location and bristle location are therefore represented topographically and temporally within the axon profile of the tract and its projection to the brain.
Keywords: Antenna; Basiconic sensilla; Embryogenesis; Locust; Topographic projections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Epithelial domains and the primordial antennal nervous system of the embryonic grasshopper Schistocerca gregaria.Invert Neurosci. 2020 Mar 26;20(2):6. doi: 10.1007/s10158-020-0240-z. Invert Neurosci. 2020. PMID: 32215732
-
Evidence for the cholinergic markers ChAT and vAChT in sensory cells of the developing antennal nervous system of the desert locust Schistocerca gregaria.Invert Neurosci. 2020 Oct 22;20(4):19. doi: 10.1007/s10158-020-00252-4. Invert Neurosci. 2020. PMID: 33090291 Free PMC article.
-
Pioneer neurons of the antennal nervous system project to protocerebral pioneers in the grasshopper Schistocerca gregaria.Dev Genes Evol. 2015 Nov;225(6):377-82. doi: 10.1007/s00427-015-0519-y. Epub 2015 Nov 9. Dev Genes Evol. 2015. PMID: 26553379
-
Dysregulation of axogenesis in the antennal nervous system of the embryonic grasshopper Schistocerca gregaria.Invert Neurosci. 2019 Jan 17;19(1):3. doi: 10.1007/s10158-019-0223-0. Invert Neurosci. 2019. PMID: 30656487
-
Patterns of cell death in the embryonic antenna of the grasshopper Schistocerca gregaria.Dev Genes Evol. 2018 Mar;228(2):105-118. doi: 10.1007/s00427-018-0607-x. Epub 2018 Mar 6. Dev Genes Evol. 2018. PMID: 29511851
References
-
- Adams RR, Maiato H, Earnshaw W, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome Segregation. J Cell Biol. 2001;153:865–879. doi: 10.1083/jcb.153.4.865. - DOI - PMC - PubMed
-
- Bentley D, Keshishian H, Shankland M, Torian-Raymond A. Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. J Embryol Exp Morphol. 1979;54:47–74. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

