Palopegteriparatide Treatment Improves Renal Function in Adults with Chronic Hypoparathyroidism: 1-Year Results from the Phase 3 PaTHway Trial
- PMID: 38691316
- PMCID: PMC11133178
- DOI: 10.1007/s12325-024-02843-8
Palopegteriparatide Treatment Improves Renal Function in Adults with Chronic Hypoparathyroidism: 1-Year Results from the Phase 3 PaTHway Trial
Abstract
Introduction: Individuals with chronic hypoparathyroidism managed with conventional therapy (active vitamin D and calcium) have an increased risk for renal dysfunction versus age- and sex-matched controls. Treatments that replace the physiologic effects of parathyroid hormone (PTH) while reducing the need for conventional therapy may help prevent a decline in renal function in this population. This post hoc analysis examined the impact of palopegteriparatide treatment on renal function in adults with chronic hypoparathyroidism.
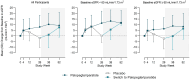
Methods: PaTHway is a phase 3 trial of palopegteriparatide in adults with chronic hypoparathyroidism that included a randomized, double-blind, placebo-controlled 26-week period followed by an ongoing 156-week open-label extension (OLE) period. Changes in renal function over 52 weeks (26 weeks blinded + 26 weeks OLE) were assessed using estimated glomerular filtration rate (eGFR). A subgroup analysis was performed with participants stratified by baseline eGFR < 60 or ≥ 60 mL/min/1.73 m2.
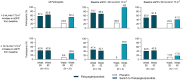
Results: At week 52, over 95% (78/82) of participants remained enrolled in the OLE and of those, 86% maintained normocalcemia and 95% achieved independence from conventional therapy (no active vitamin D and ≤ 600 mg/day of calcium), with none requiring active vitamin D. Treatment with palopegteriparatide over 52 weeks resulted in a mean (SD) increase in eGFR of 9.3 (11.7) mL/min/1.73 m2 from baseline (P < 0.0001) and 43% of participants had an increase ≥ 10 mL/min/1.73 m2. In participants with baseline eGFR < 60 mL/min/1.73 m2, 52 weeks of treatment with palopegteriparatide resulted in a mean (SD) increase of 11.5 (11.3) mL/min/1.73 m2 (P < 0.001). One case of nephrolithiasis was reported for a participant in the placebo group during blinded treatment; none were reported through week 52 with palopegteriparatide.
Conclusion: In this post hoc analysis of the PaTHway trial, palopegteriparatide treatment was associated with significantly improved eGFR at week 52 in addition to previously reported maintenance and normalization of serum and urine biochemistries. Further investigation of palopegteriparatide for the preservation of renal function in hypoparathyroidism is warranted.
Trial registration: ClinicalTrials.gov NCT04701203.
Keywords: Clinical trial; Glomerular filtration rate; Hypoparathyroidism; Kidney; Palopegteriparatide; Parathyroid hormone; Renal disease; TransCon PTH.
Plain language summary
Chronic hypoparathyroidism is caused by inadequate parathyroid hormone (PTH) levels. Hypoparathyroidism is managed with conventional therapy (active vitamin D and calcium), but over time the disease itself and conventional therapy can increase the risk of medical complications including kidney problems. This study looked at how a new treatment for chronic hypoparathyroidism, palopegteriparatide (approved in the European Union under the brand name YORVIPATH®), affects kidney function in adults in the PaTHway clinical trial. Participants were randomly assigned to receive palopegteriparatide or a placebo injection once daily along with conventional therapy. For both groups, clinicians used a protocol to eliminate conventional therapy while maintaining normal blood calcium levels. After 26 weeks, participants on placebo switched to palopegteriparatide. Ninety-five percent of participants were still enrolled in the PaTHway trial after 52 weeks. Of those, 86% had normal blood calcium levels and 95% did not need conventional therapy (not taking vitamin D and not taking therapeutic doses of calcium [> 600 mg/day]). After 52 weeks of treatment with palopegteriparatide, significant improvements were seen in a measure of kidney function called estimated glomerular filtration rate (eGFR). Improvements in eGFR from the beginning of the trial to week 52 were considered clinically meaningful for over 57% of participants. In participants with impaired kidney function at the beginning of the trial, eGFR improvements were even greater, and 74% of participants had a clinically meaningful improvement. These results suggest that palopegteriparatide treatment may be beneficial for kidney function in adults with chronic hypoparathyroidism, especially those with impaired kidney function.
© 2024. The Author(s).
Conflict of interest statement
Lars Rejnmark: Research funding from Takeda, Kyowa Kirin International, Ascendis, and Calcilytix; honoraria from Calcilytix Therapeutics; advisory board for Takeda and Amolyt. Elvira Gosmanova: Honoraria from Novo Nordisk and Takeda; advisory board role for Ascendis Pharma and Takeda; travel, accommodations, and expenses from Ascendis Pharma; industry-sponsored grants from AstraZeneca, AbbVie, and Kowa Research Institute. Aliya Khan: Research funding and/or industry grants from Amolyt, Ascendis, Chugai, Radius, and Takeda; honoraria from and advisory board member for Amgen, Alexion, Ascendis, and Takeda; travel, accommodations, and expenses from Ascendis; consulting role for Amgen, Alexion, Amolyt, and Ascendis; speakers bureau participation for Amgen. Noriko Makita: Research funding from Kyowa Kirin. Yasuo Imanishi: speakers bureau for Kyowa Kirin Co., Ltd and Daiichi Sankyo Co., Ltd. Yasuhiro Takeuchi: Honoraria from Chugai Pharmaceutical Co. Ltd; consulting fee from Teijin Pharma; speakers bureau for Amgen Japan. Stuart Sprague: Research funding from Amgen, Amylot, Ascendis, Ardelyx, Frensinius, and OPKO and consultant Amgen, Ardelyx, Bayer, Fresenius, Horizon, OPKO, and Shire. Dolores Shoback: Research/salary funding from Bone Health Tech; research funding from Ascendis. Lynn Kohlmeier: Research funding from Amolyt and Ascendis; speakers bureau, honoraria from Amgen, Radius, and Ascendis; advisory board, consultant for Radius, Alexion, and Ascendis. Mishaela Rubin: Study investigator for Takeda, Ascendis, Amolyt, and Calcilytix; advisory board for Ascendis, speakers bureau for Ascendis; consulting for MBX. Andrea Palermo: Consultant for Theramex, Bruno Farmaceutici, Amgen; research funding from Amgen, Shire, Ascendis; speakers bureau UCB, Amgen; industry grant from Amgen. Peter Schwarz: Stock ownership Novo Nordisk, Genmab. Claudia Gagnon: Advisory board member for Novo Nordisk; honoraria from Amgen; industry grants from Ascendis, Shire, and Takeda. Elena Tsourdi: Honoraria from Amgen, UCB, Takeda, Kyowa Kirin, and Ascendis; advisory board member for Amgen and UCB. Carol Zhao, Michael Makara, Michael Ominsky, Bryant Lai, Jenny Ukena, Christopher Sibley, and Aimee Shu are full-time employees of Ascendis Pharma.
Figures
References
-
- Gosmanova EO, Houillier P, Rejnmark L, Marelli C, Bilezikian JP. Renal complications in patients with chronic hypoparathyroidism on conventional therapy: a systematic literature review: renal disease in chronic hypoparathyroidism. Rev Endocr Metab Disord. 2021;22(2):297–316. doi: 10.1007/s11154-020-09613-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

