Real-World Gait Detection Using a Wrist-Worn Inertial Sensor: Validation Study
- PMID: 38691395
- PMCID: PMC11097052
- DOI: 10.2196/50035
Real-World Gait Detection Using a Wrist-Worn Inertial Sensor: Validation Study
Abstract
Background: Wrist-worn inertial sensors are used in digital health for evaluating mobility in real-world environments. Preceding the estimation of spatiotemporal gait parameters within long-term recordings, gait detection is an important step to identify regions of interest where gait occurs, which requires robust algorithms due to the complexity of arm movements. While algorithms exist for other sensor positions, a comparative validation of algorithms applied to the wrist position on real-world data sets across different disease populations is missing. Furthermore, gait detection performance differences between the wrist and lower back position have not yet been explored but could yield valuable information regarding sensor position choice in clinical studies.
Objective: The aim of this study was to validate gait sequence (GS) detection algorithms developed for the wrist position against reference data acquired in a real-world context. In addition, this study aimed to compare the performance of algorithms applied to the wrist position to those applied to lower back-worn inertial sensors.
Methods: Participants with Parkinson disease, multiple sclerosis, proximal femoral fracture (hip fracture recovery), chronic obstructive pulmonary disease, and congestive heart failure and healthy older adults (N=83) were monitored for 2.5 hours in the real-world using inertial sensors on the wrist, lower back, and feet including pressure insoles and infrared distance sensors as reference. In total, 10 algorithms for wrist-based gait detection were validated against a multisensor reference system and compared to gait detection performance using lower back-worn inertial sensors.
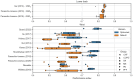
Results: The best-performing GS detection algorithm for the wrist showed a mean (per disease group) sensitivity ranging between 0.55 (SD 0.29) and 0.81 (SD 0.09) and a mean (per disease group) specificity ranging between 0.95 (SD 0.06) and 0.98 (SD 0.02). The mean relative absolute error of estimated walking time ranged between 8.9% (SD 7.1%) and 32.7% (SD 19.2%) per disease group for this algorithm as compared to the reference system. Gait detection performance from the best algorithm applied to the wrist inertial sensors was lower than for the best algorithms applied to the lower back, which yielded mean sensitivity between 0.71 (SD 0.12) and 0.91 (SD 0.04), mean specificity between 0.96 (SD 0.03) and 0.99 (SD 0.01), and a mean relative absolute error of estimated walking time between 6.3% (SD 5.4%) and 23.5% (SD 13%). Performance was lower in disease groups with major gait impairments (eg, patients recovering from hip fracture) and for patients using bilateral walking aids.
Conclusions: Algorithms applied to the wrist position can detect GSs with high performance in real-world environments. Those periods of interest in real-world recordings can facilitate gait parameter extraction and allow the quantification of gait duration distribution in everyday life. Our findings allow taking informed decisions on alternative positions for gait recording in clinical studies and public health.
Trial registration: ISRCTN Registry 12246987; https://www.isrctn.com/ISRCTN12246987.
International registered report identifier (irrid): RR2-10.1136/bmjopen-2021-050785.
Keywords: Mobilise-D; accelerometer; digital health; digital mobility outcomes; inertial measurement unit; validation; walking; wearable sensor.
©Felix Kluge, Yonatan E Brand, M Encarna Micó-Amigo, Stefano Bertuletti, Ilaria D'Ascanio, Eran Gazit, Tecla Bonci, Cameron Kirk, Arne Küderle, Luca Palmerini, Anisoara Paraschiv-Ionescu, Francesca Salis, Abolfazl Soltani, Martin Ullrich, Lisa Alcock, Kamiar Aminian, Clemens Becker, Philip Brown, Joren Buekers, Anne-Elie Carsin, Marco Caruso, Brian Caulfield, Andrea Cereatti, Lorenzo Chiari, Carlos Echevarria, Bjoern Eskofier, Jordi Evers, Judith Garcia-Aymerich, Tilo Hache, Clint Hansen, Jeffrey M Hausdorff, Hugo Hiden, Emily Hume, Alison Keogh, Sarah Koch, Walter Maetzler, Dimitrios Megaritis, Martijn Niessen, Or Perlman, Lars Schwickert, Kirsty Scott, Basil Sharrack, David Singleton, Beatrix Vereijken, Ioannis Vogiatzis, Alison Yarnall, Lynn Rochester, Claudia Mazzà, Silvia Del Din, Arne Mueller. Originally published in JMIR Formative Research (https://formative.jmir.org), 01.05.2024.
Conflict of interest statement
Conflicts of Interest: AM and FK are employees of and may hold stock in Novartis. BE reports consulting activities with adidas AG, Siemens AG, Siemens Healthineers AG, and WSAudiology GmbH outside of the study. BE is a shareholder in Portabiles HealthCare Technologies GmbH. In addition, BE holds a patent related to gait assessment. LP and LC are cofounders and own shares of mHealth Technologies. LS and CB are consultants of Philipps Healthcare, Bosch Healthcare, Eli Lilly, and Gait-up. MN is an employee of McRoberts. SD and JMH report consultancy activity with Hoffmann-La Roche Ltd outside of this study.
Figures



References
-
- Schlachetzki JCM, Barth J, Marxreiter F, Gossler J, Kohl Z, Reinfelder S, Gassner H, Aminian K, Eskofier BM, Winkler J, Klucken J. Wearable sensors objectively measure gait parameters in Parkinson's disease. PLoS One. 2017;12(10):e0183989. doi: 10.1371/journal.pone.0183989. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0183989 PONE-D-17-06837 - DOI - PMC - PubMed
-
- van den Berg-Emons HR, Bussmann JH, Balk A, Keijzer-Oster D, Stam H. Level of activities associated with mobility during everyday life in patients with chronic congestive heart failure as measured with an "activity monitor". Phys Ther. 2001;81(9):1502–1511. doi: 10.1093/ptj/81.9.1502. https://academic.oup.com/ptj/article/81/9/1502/2857610?login=false 2857610 - DOI - PubMed
-
- Warmerdam E, Hausdorff JM, Atrsaei A, Zhou Y, Mirelman A, Aminian K, Espay AJ, Hansen C, Evers LJW, Keller A, Lamoth C, Pilotto A, Rochester L, Schmidt G, Bloem BR, Maetzler W. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020;19(5):462–470. doi: 10.1016/S1474-4422(19)30397-7.S1474-4422(19)30397-7 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

