Catalysis driven by an amyloid-substrate complex
- PMID: 38691589
- PMCID: PMC11087796
- DOI: 10.1073/pnas.2314704121
Catalysis driven by an amyloid-substrate complex
Abstract
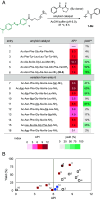
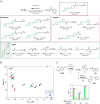
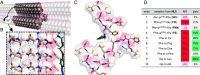
Amine modification through nucleophilic attack of the amine functionality is a very common chemical transformation. Under biorelevant conditions using acidic-to-neutral pH buffer, however, the nucleophilic reaction of alkyl amines (pKa ≈ 10) is not facile due to the generation of ammonium ions lacking nucleophilicity. Here, we disclose a unique molecular transformation system,
Keywords: amyloid; amyloid catalyst; catalysis; nucleophilic reaction.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Liu X., et al. , The structural basis of protein acetylation by the P300/Cbp transcriptional coactivator. Nature 451, 846–850 (2008). - PubMed
-
- Liao L., et al. , Structural and molecular dynamics analysis of plant serotonin N-acetyltransferase reveal an acid/base-assisted catalysis in melatonin biosynthesis. Angew. Chem. Int. Ed. 60, 12020–12026 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

