Improved Enzymatic Production of the Fucosylated Human Milk Oligosaccharide LNFP II with GH29B α-1,3/4-l-Fucosidases
- PMID: 38691641
- PMCID: PMC11100010
- DOI: 10.1021/acs.jafc.4c01547
Improved Enzymatic Production of the Fucosylated Human Milk Oligosaccharide LNFP II with GH29B α-1,3/4-l-Fucosidases
Erratum in
-
Correction to "Improved Enzymatic Production of the Fucosylated Human Milk Oligosaccharide LNFP II with GH29B α-1,3/4-L-Fucosidases".J Agric Food Chem. 2024 Jun 26;72(25):14480. doi: 10.1021/acs.jafc.4c04966. Epub 2024 Jun 14. J Agric Food Chem. 2024. PMID: 38876976 Free PMC article. No abstract available.
Abstract
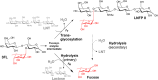
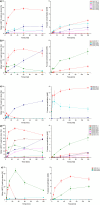
Five GH29B α-1,3/4-l-fucosidases (EC 3.2.1.111) were investigated for their ability to catalyze the formation of the human milk oligosaccharide lacto-N-fucopentaose II (LNFP II) from lacto-N-tetraose (LNT) and 3-fucosyllactose (3FL) via transglycosylation. We studied the effect of pH on transfucosylation and hydrolysis and explored the impact of specific mutations using molecular dynamics simulations. LNFP II yields of 91 and 65% were obtained for the wild-type SpGH29C and CpAfc2 enzymes, respectively, being the highest LNFP II transglycosylation yields reported to date. BbAfcB and BiAfcB are highly hydrolytic enzymes. The results indicate that the effects of pH and buffer systems are enzyme-dependent yet relevant to consider when designing transglycosylation reactions. Replacing Thr284 in BiAfcB with Val resulted in increased transglycosylation yields, while the opposite replacement of Val258 in SpGH29C and Val289 CpAfc2 with Thr decreased the transfucosylation, confirming a role of Thr and Val in controlling the flexibility of the acid/base loop in the enzymes, which in turn affects transglycosylation. The substitution of an Ala residue with His almost abolished secondary hydrolysis in CpAfc2 and BbAfcB. The results are directly applicable in the enhancement of transglycosylation and may have significant implications for manufacturing of LNFP II as a new infant formula ingredient.
Keywords: acid/base residue flexibility; molecular dynamics; protein engineering; transglycosylation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

