Impact of hematopoietic cell transplantation on myocardial fibrosis in young patients with sickle cell disease
- PMID: 38691679
- PMCID: PMC11347799
- DOI: 10.1182/blood.2023023028
Impact of hematopoietic cell transplantation on myocardial fibrosis in young patients with sickle cell disease
Abstract
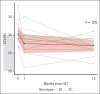
Serial cardiovascular magnetic resonance evaluation of children and young adults with SCD who underwent hematopoietic cell transplantation showed mean ECV, representing diffuse myocardial fibrosis, decreased 3.4% from baseline to 12 months posttransplantation. This trial was registered at www.clinicaltrials.gov as #NCT04362293.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
A.S. has received consultant fees from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine, is a medical monitor for the Conditioning SCID Infants Diagnosed Early (CSIDE) clinical trial for which he receives financial compensation, and has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. A.S. is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. has no direct financial interest in these therapies. S.G. is a coinventor on patent applications in the fields of cell or gene therapy for cancer, a member of the Scientific Advisory Board of Be Biopharma and CARGO, a member of the Data and Safety Monitoring Board of Immatics, and has received honoraria from TESSA Therapeutics within the past year. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Curative therapies raise the bar for the heart.Blood. 2024 Aug 8;144(6):597-599. doi: 10.1182/blood.2024025187. Blood. 2024. PMID: 39115829 No abstract available.
References
-
- Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387(10037):2565–2574. - PubMed
-
- Morin CE, Sharma A, Selukar S, et al. Diffuse myocardial fibrosis occurs in young patients with sickle cell anemia despite early disease-modifying therapy. Blood. 2023;141(11):1358–1362. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

