Serial Postoperative Circulating Tumor DNA Assessment Has Strong Prognostic Value During Long-Term Follow-Up in Patients With Breast Cancer
- PMID: 38691816
- PMCID: PMC11161241
- DOI: 10.1200/PO.23.00456
Serial Postoperative Circulating Tumor DNA Assessment Has Strong Prognostic Value During Long-Term Follow-Up in Patients With Breast Cancer
Erratum in
-
Erratum: Serial Postoperative Circulating Tumor DNA Assessment Has Strong Prognostic Value During Long-Term Follow-Up in Patients With Breast Cancer.JCO Precis Oncol. 2024 Jun;8:e2400310. doi: 10.1200/PO.24.00310. JCO Precis Oncol. 2024. PMID: 38837747 Free PMC article. No abstract available.
Abstract
Purpose: Here, we report the sensitivity of a personalized, tumor-informed circulating tumor DNA (ctDNA) assay (Signatera) for detection of molecular relapse during long-term follow-up of patients with breast cancer.
Methods: A total of 156 patients with primary breast cancer were monitored clinically for up to 12 years after surgery and adjuvant chemotherapy. Semiannual blood samples were prospectively collected, and analyzed retrospectively to detect residual disease by ultradeep sequencing using ctDNA assays, developed from primary tumor whole-exome sequencing data.
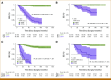
Results: Personalized Signatera assays detected ctDNA ahead of clinical or radiologic relapse in 30 of the 34 patients who relapsed (patient-level sensitivity of 88.2%). Relapse was predicted with a lead interval of up to 38 months (median, 10.5 months; range, 0-38 months), and ctDNA positivity was associated with shorter relapse-free survival (P < .0001) and overall survival (P < .0001). All relapsing triple-negative patients (n = 7/23) had a ctDNA-positive test within a median of 8 months (range, 0-19 months), while the 16 nonrelapsed patients with triple-negative breast cancer remained ctDNA-negative during a median follow-up of 58 months (range, 8-99 months). The four patients who had negative tests before relapse all had hormone receptor-positive (HR+) disease and conversely, five of the 122 nonrelapsed patients (all HR+) had an occasional positive test.
Conclusion: Serial postoperative ctDNA assessment has strong prognostic value, provides a potential window for earlier therapeutic intervention, and may enable more effective monitoring than current clinical tests such as cancer antigen 15-3. Our study provides evidence that those with serially negative ctDNA tests have superior clinical outcomes, providing reassurance to patients with breast cancer. For select cases with HR+ disease, decisions about treatment management might require serial monitoring despite the ctDNA-positive result.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Gradishar WJ, Moran MS, Abraham J, et al. : NCCN Guidelines® insights: Breast cancer, version 4.2021. J Natl Compr Canc Netw 19:484-493, 2021 - PubMed
-
- Senkus E, Kyriakides S, Ohno S, et al. : Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v8-v30, 2015 - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group : Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

