Allergen-Encapsulating Nanoparticles Reprogram Pathogenic Allergen-Specific Th2 Cells to Suppress Food Allergy
- PMID: 38691819
- PMCID: PMC11527797
- DOI: 10.1002/adhm.202400237
Allergen-Encapsulating Nanoparticles Reprogram Pathogenic Allergen-Specific Th2 Cells to Suppress Food Allergy
Abstract
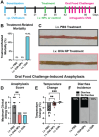
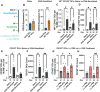
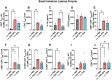
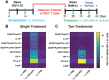
Food allergy is a prevalent, potentially deadly disease caused by inadvertent sensitization to benign food antigens. Pathogenic Th2 cells are a major driver for disease, and allergen-specific immunotherapies (AIT) aim to increase the allergen threshold required to elicit severe allergic symptoms. However, the majority of AIT approaches require lengthy treatments and convey transient disease suppression, likely due to insufficient targeting of pathogenic Th2 responses. Here, the ability of allergen-encapsulating nanoparticles to directly suppress pathogenic Th2 responses and reactivity is investigated in a mouse model of food allergy. NPs associate with pro-tolerogenic antigen presenting cells, provoking accumulation of antigen-specific, functionally suppressive regulatory T cells in the small intestine lamina propria. Two intravenous doses of allergen encapsulated in poly(lactide-co-glycolide) nanoparticles (NPs) significantly reduces oral food challenge (OFC)-induced anaphylaxis. Importantly, NP treatment alters the fates of pathogenic allergen-specific Th2 cells, reprogramming these cells toward CD25+FoxP3+ regulatory and CD73+FR4+ anergic phenotypes. NP-mediated reductions in the frequency of effector cells in the gut and mast cell degranulation following OFC are also demonstrated. These studies reveal mechanisms by which an allergen-encapsulating NP therapy and, more broadly, allergen-specific immunotherapies, can rapidly attenuate allergic responses by targeting pathogenic Th2 cells.
Keywords: allergen‐specific Th2 cells; allergen‐specific immunotherapy mechanisms; biomaterials; polymeric nanoparticles; regulatory T cells.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
L.D.S. and S.D.M. are consultants for Cour Pharmaceutical Development Company, Inc., and have financial interests in the nanoparticle technology described in this manuscript. J.J.P. is employed by Cour Pharmaceutical Development Company, Inc. All other authors declare they have no conflict of interest. The nanoparticle technology used here is reflective of that described in patent US‐20150190485.
Figures
References
-
- Gupta R. S., Springston E. E., Warrier M. R., Smith B., Kumar R., Pongracic J., Holl J. L., Pediatrics. 2011, 128, e9. - PubMed
-
- Schoos A.‐M. M., Bullens D., Chawes B. L., Costa J., De Vlieger L., DunnGalvin A., Epstein M. M., Garssen J., Hilger C., Knipping K., Kuehn A., Mijakoski D., Nekliudov D. MunbN., Ozdemir C., Patient K., Peroni D., Stoleski S., Stylianou E., Tukalj M., Verhoeckx K., Zidarn M., van de Veen W., Front. Immunol. 2020, 11, 568598. - PMC - PubMed
-
- PALISADE , N. Engl. J. Med. 2018, 379, 1991. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

