Treatment with inhaled Argon: a systematic review of pre-clinical and clinical studies with meta-analysis on neuroprotective effect
- PMID: 38691938
- PMCID: PMC11070688
- DOI: 10.1016/j.ebiom.2024.105143
Treatment with inhaled Argon: a systematic review of pre-clinical and clinical studies with meta-analysis on neuroprotective effect
Abstract
Background: Argon (Ar) has been proposed as a potential therapeutic agent in multiple clinical conditions, specifically in organ protection. However, conflicting data on pre-clinical models, together with a great variability in Ar administration protocols and outcome assessments, have been reported. The aim of this study was to review evidence on treatment with Ar, with an extensive investigation on its neuroprotective effect, and to summarise all tested administration protocols.
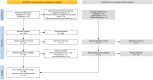
Methods: Using the PubMed database, all existing pre-clinical and clinical studies on the treatment with Ar were systematically reviewed (registration: https://doi.org/10.17605/OSF.IO/7983D). Study titles and abstracts were screened, extracting data from relevant studies post full-text review. Exclusion criteria included absence of full text and non-English language. Furthermore, meta-analysis was also performed to assess Ar potential as neuroprotectant agent in different clinical conditions: cardiac arrest, traumatic brain injury, ischemic stroke, perinatal hypoxic-ischemic encephalopathy, subarachnoid haemorrhage. Standardised mean differences for neurological, cognitive and locomotor, histological, and physiological measures were evaluated, through appropriate tests, clinical, and laboratory variables. In vivo studies were evaluated for risk of bias using the Systematic Review Center for Laboratory Animal Experimentation tool, while in vitro studies underwent assessment with a tool developed by the Office of Health Assessment and Translation.
Findings: The systematic review detected 60 experimental studies (16 in vitro, 7 ex vivo, 31 in vivo, 6 with both in vitro and in vivo) investigating the role of Ar. Only one clinical study was found. Data from six in vitro and nineteen in vivo studies were included in the meta-analyses. In pre-clinical models, Ar administration resulted in improved neurological, cognitive and locomotor, and histological outcomes without any change in physiological parameters (i.e., absence of adverse events).
Interpretation: This systematic review and meta-analysis based on experimental studies supports the neuroprotective effect of Ar, thus providing a rationale for potential translation of Ar treatment in humans. Despite adherence to established guidelines and methodologies, limitations in data availability prevented further analyses to investigate potential sources of heterogeneity due to study design.
Funding: This study was funded in part by Italian Ministry of Health-Current researchIRCCS and by Ministero della Salute Italiano, Ricerca Finalizzata, project no. RF 2019-12371416.
Keywords: Argon; Meta-analysis; Neuroprotection; Noble gas; Organ protection.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests G.G received funding from Fischer&Paykel, MSD, Pfizer, and received fees from Getinge, Draeger Medical, Cook, MundiPharma, Fischer&Paykel, Pfizer. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures








References
-
- Magliocca A., Fries M. Inhaled gases as novel neuroprotective therapies in the postcardiac arrest period. Curr Opin Crit Care. 2021;27(3):255–260. - PubMed

