A body-brain circuit that regulates body inflammatory responses
- PMID: 38692285
- PMCID: PMC11186780
- DOI: 10.1038/s41586-024-07469-y
A body-brain circuit that regulates body inflammatory responses
Abstract
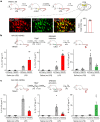
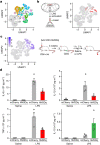
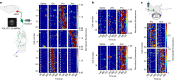
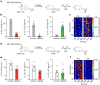
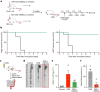
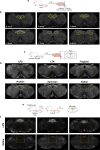
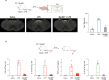
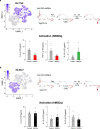
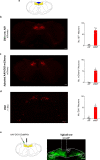
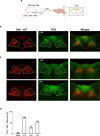
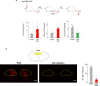
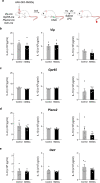
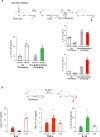
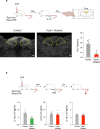
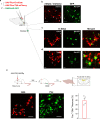
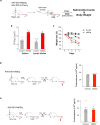
The body-brain axis is emerging as a principal conductor of organismal physiology. It senses and controls organ function1,2, metabolism3 and nutritional state4-6. Here we show that a peripheral immune insult strongly activates the body-brain axis to regulate immune responses. We demonstrate that pro-inflammatory and anti-inflammatory cytokines communicate with distinct populations of vagal neurons to inform the brain of an emerging inflammatory response. In turn, the brain tightly modulates the course of the peripheral immune response. Genetic silencing of this body-brain circuit produced unregulated and out-of-control inflammatory responses. By contrast, activating, rather than silencing, this circuit affords neural control of immune responses. We used single-cell RNA sequencing, combined with functional imaging, to identify the circuit components of this neuroimmune axis, and showed that its selective manipulation can effectively suppress the pro-inflammatory response while enhancing an anti-inflammatory state. The brain-evoked transformation of the course of an immune response offers new possibilities in the modulation of a wide range of immune disorders, from autoimmune diseases to cytokine storm and shock.
© 2024. The Author(s).
Conflict of interest statement
H.J. and C.S.Z. are co-inventors in a patent application describing this work. C.S.Z. is a scientific co-founder of Kallyope, Cajal Neurosciences and Nilo. All other authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

