Efficacy of psilocybin for treating symptoms of depression: systematic review and meta-analysis
- PMID: 38692686
- PMCID: PMC11062320
- DOI: 10.1136/bmj-2023-078084
Efficacy of psilocybin for treating symptoms of depression: systematic review and meta-analysis
Erratum in
-
CORRECTION: Efficacy of psilocybin for treating symptoms of depression: systematic review and meta-analysis.BMJ. 2025 Feb 10;388:r111. doi: 10.1136/bmj.r111. BMJ. 2025. PMID: 39929528 Free PMC article. No abstract available.
Expression of concern in
-
EXPRESSION OF CONCERN: Efficacy of psilocybin for treating symptoms of depression: systematic review and meta-analysis.BMJ. 2024 May 4;385:q1025. doi: 10.1136/bmj.q1025. BMJ. 2024. PMID: 38704154 No abstract available.
Abstract
Objective: To determine the efficacy of psilocybin as an antidepressant compared with placebo or non-psychoactive drugs.
Design: Systematic review and meta-analysis.
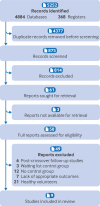
Data sources: Five electronic databases of published literature (Cochrane Central Register of Controlled Trials, Medline, Embase, Science Citation Index and Conference Proceedings Citation Index, and PsycInfo) and four databases of unpublished and international literature (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, ProQuest Dissertations and Theses Global, and PsycEXTRA), and handsearching of reference lists, conference proceedings, and abstracts.
Data synthesis and study quality: Information on potential treatment effect moderators was extracted, including depression type (primary or secondary), previous use of psychedelics, psilocybin dosage, type of outcome measure (clinician rated or self-reported), and personal characteristics (eg, age, sex). Data were synthesised using a random effects meta-analysis model, and observed heterogeneity and the effect of covariates were investigated with subgroup analyses and metaregression. Hedges’ g was used as a measure of treatment effect size, to account for small sample effects and substantial differences between the included studies’ sample sizes. Study quality was appraised using Cochrane’s Risk of Bias 2 tool, and the quality of the aggregated evidence was evaluated using GRADE guidelines.
Eligibility criteria: Randomised trials in which psilocybin was administered as a standalone treatment for adults with clinically significant symptoms of depression and change in symptoms was measured using a validated clinician rated or self-report scale. Studies with directive psychotherapy were included if the psychotherapeutic component was present in both experimental and control conditions. Participants with depression regardless of comorbidities (eg, cancer) were eligible.
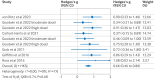
Results: Meta-analysis on 436 participants (228 female participants), average age 36-60 years, from seven of the nine included studies showed a significant benefit of psilocybin (Hedges’ g=0.66, 95% confidence interval (CI) 0.46 to 0.86, P<0.001) on change in depression scores compared with comparator treatment. Exploratory subgroup analyses and metaregressions indicated that having secondary depression (Hedges’ g=0.88, 95% CI 0.42 to 1.33), being assessed with self-report depression scales such as the Beck depression inventory (0.88, 0.42 to 1.33), and older age and previous use of psychedelics (metaregression coefficient 0.13, 95% CI 0.02 to 0.23 and 6.00, 2.48 to 9.53, respectively) were correlated with greater improvements in symptoms. All studies had a moderate risk of bias, but the change from baseline metric was associated with low heterogeneity and a statistically non-significant risk of small study bias, resulting in a moderate certainty of evidence rating.
Conclusion: Treatment effects of psilocybin were significantly larger among patients with secondary depression, when self-report scales were used to measure symptoms of depression, and when participants had previously used psychedelics. Further research is thus required to delineate the influence of expectancy effects, moderating factors, and treatment delivery on the efficacy of psilocybin as an antidepressant.
Systematic review registration: PROSPERO CRD42023388065.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; AMM is employed by IDEA Pharma, which does consultancy work for pharmaceutical companies developing drugs for physical and mental health conditions; MC was the supervisor for AMM’s University of Oxford MSc dissertation, which forms the basis for this paper; no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- World Health Organization. Depressive Disorder (Depression); 2023. https://www.who.int/news-room/fact-sheets/detail/depression .
-
- James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
