Evaluating the effect of a mobile-based symptom monitoring system for improving physical function in patients with cancer during chemotherapy: study protocol for a multicentre randomised controlled trial
- PMID: 38692724
- PMCID: PMC11086447
- DOI: 10.1136/bmjopen-2023-080976
Evaluating the effect of a mobile-based symptom monitoring system for improving physical function in patients with cancer during chemotherapy: study protocol for a multicentre randomised controlled trial
Abstract
Introduction: Symptoms due to chemotherapy are common in patients with cancer. Cancer-related symptoms are closely associated with the deterioration of physical function which can be associated with decreased quality of life and increased mortality. Thus, timely symptom identification is critical for improving cancer prognosis and survival. Recently, remote symptom monitoring system using digital technology has demonstrated its effects on symptom control or survival. However, few studies examined whether remote monitoring would contribute to retaining physical function among patients with cancer. Therefore, this study aimed to evaluate the effectiveness of mobile-based symptom monitoring in improving physical function among patients with cancer under chemotherapy.
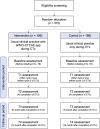
Methods and analysis: This study is a multicentre, open-label, parallel-group, randomised controlled trial. We will recruit 372 patients at three tertiary hospitals located in Seoul, South Korea. Study participants will be randomly assigned to either an intervention group receiving the ePRO-CTCAE app and a control group receiving routine clinical practice only. The primary outcome is changes in physical function from commencement to completion of planned chemotherapy. A linear mixed model will be performed under the intention-to-treat principle. The secondary outcomes include physical activity level; changes in pain interference; changes in depressive symptom; unplanned clinical visits; additional medical expenditure for symptom management; completion rate of planned chemotherapy; changes in symptom burden and health-related quality of life; and 1-year overall mortality.
Ethics and dissemination: The study has been approved by the institutional review board and ethics committee at the three university hospitals involved in this trial. Written informed consent will be obtained from all the participants. The results of the trial will be submitted for publication in peer-reviewed academic journals and disseminated through relevant literatures.
Trial registration number: KCT0007220.
Keywords: CHEMOTHERAPY; Patient-Centered Care; Protocols & guidelines; Randomized Controlled Trial; eHealth.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Patient Reported Outcome Measures in cancer care: a hybrid effectiveness-Implementation trial to optimise Symptom control and health service Experience (PROMISE)-protocol for a randomised controlled trial of electronic self-reporting of symptoms versus usual care during and following treatment in patients with cancer.BMJ Open. 2024 Nov 19;14(11):e090836. doi: 10.1136/bmjopen-2024-090836. BMJ Open. 2024. PMID: 39566946 Free PMC article.
-
The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer.BMJ Open. 2017 Jun 6;7(5):e015016. doi: 10.1136/bmjopen-2016-015016. BMJ Open. 2017. PMID: 28592577 Free PMC article. Clinical Trial.
-
SYMptom monitoring with Patient-Reported Outcomes using a web application among patients with Lung cancer in the Netherlands (SYMPRO-Lung): study protocol for a stepped-wedge randomised controlled trial.BMJ Open. 2021 Sep 13;11(9):e052494. doi: 10.1136/bmjopen-2021-052494. BMJ Open. 2021. PMID: 34518276 Free PMC article.
-
NAVIGATE: improving survival in vulnerable patients with lung cancer through nurse navigation, symptom monitoring and exercise - study protocol for a multicentre randomised controlled trial.BMJ Open. 2022 Oct 31;12(10):e060242. doi: 10.1136/bmjopen-2021-060242. BMJ Open. 2022. PMID: 36316074 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
References
-
- Itano JK, Brant JM, Conde F, et al. . Core Curriculum for Oncology. Nursing-E-Book: Elsevier Health Sciences, 2015.
-
- Craven O, Hughes CA, Burton A, et al. . Is a nurse-led telephone intervention a viable alternative to nurse-led home care and standard care for patients receiving oral capecitabine? results from a large prospective audit in patients with colorectal cancer. Eur J Cancer Care (Engl) 2013;22:413–9. 10.1111/ecc.12047 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
