Novel point-of-care cytokine biomarker lateral flow test for the screening for sexually transmitted infections and bacterial vaginosis: study protocol of a multicentre multidisciplinary prospective observational clinical study to evaluate the performance and feasibility of the Genital InFlammation Test (GIFT)
- PMID: 38692732
- PMCID: PMC11086546
- DOI: 10.1136/bmjopen-2024-084918
Novel point-of-care cytokine biomarker lateral flow test for the screening for sexually transmitted infections and bacterial vaginosis: study protocol of a multicentre multidisciplinary prospective observational clinical study to evaluate the performance and feasibility of the Genital InFlammation Test (GIFT)
Abstract
Introduction: A prototype lateral flow device detecting cytokine biomarkers interleukin (IL)-1α and IL-1β has been developed as a point-of-care test-called the Genital InFlammation Test (GIFT)-for detecting genital inflammation associated with sexually transmitted infections (STIs) and/or bacterial vaginosis (BV) in women. In this paper, we describe the rationale and design for studies that will be conducted in South Africa, Zimbabwe and Madagascar to evaluate the performance of GIFT and how it could be integrated into routine care.
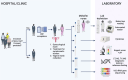
Methods and analysis: We will conduct a prospective, multidisciplinary, multicentre, cross-sectional and observational clinical study comprising two distinct components: a biomedical ('diagnostic study') and a qualitative, modelling and economic ('an integration into care study') part. The diagnostic study aims to evaluate GIFT's performance in identifying asymptomatic women with discharge-causing STIs (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV) and Mycoplasma genitalium (MG)) and BV. Study participants will be recruited from women attending research sites and family planning services. Several vaginal swabs will be collected for the evaluation of cytokine concentrations (ELISA), STIs (nucleic acid amplification tests), BV (Nugent score) and vaginal microbiome characteristics (16S rRNA gene sequencing). The first collected vaginal swab will be used for the GIFT assay which will be performed in parallel by a healthcare worker in the clinic near the participant, and by a technician in the laboratory. The integration into care study aims to explore how GIFT could be integrated into routine care. Four activities will be conducted: user experiences and/or perceptions of the GIFT device involving qualitative focus group discussions and in-depth interviews with key stakeholders; discrete choice experiments; development of a decision tree classification algorithm; and economic evaluation of defined management algorithms.
Ethics and dissemination: Findings will be reported to participants, collaborators and local government for the three sites, presented at national and international conferences, and disseminated in peer-reviewed publications.The protocol and all study documents such as informed consent forms were reviewed and approved by the University of Cape Town Human Research Ethics Committee (HREC reference 366/2022), Medical Research Council of Zimbabwe (MRCZ/A/2966), Comité d'Ethique pour la Recherche Biomédicale de Madagascar (N° 143 MNSAP/SG/AMM/CERBM) and the London School of Hygiene and Tropical Medicine ethics committee (LSHTM reference 28046).Before the start, this study was submitted to the Clinicaltrials.gov public registry (NCT05723484).
Trial registration number: NCT05723484.
Keywords: bacteriology; epidemiology; public health; sexually transmitted disease.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The last authors, J-AP and LM, declare sharing a patent for the biomarkers for GIFT: patent number PCT/IB 2014/065740, October 2014.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
