Safety and immunogenicity of a polyvalent DNA-protein HIV vaccine with matched Env immunogens delivered as a prime-boost regimen or coadministered in HIV-uninfected adults in the USA (HVTN 124): a phase 1, placebo-controlled, double-blind randomised controlled trial
- PMID: 38692824
- PMCID: PMC11228966
- DOI: 10.1016/S2352-3018(24)00036-5
Safety and immunogenicity of a polyvalent DNA-protein HIV vaccine with matched Env immunogens delivered as a prime-boost regimen or coadministered in HIV-uninfected adults in the USA (HVTN 124): a phase 1, placebo-controlled, double-blind randomised controlled trial
Erratum in
-
Correction to Lancet HIV 2024; 11: e285-99.Lancet HIV. 2024 Dec;11(12):e804. doi: 10.1016/S2352-3018(24)00152-8. Epub 2024 May 31. Lancet HIV. 2024. PMID: 38830377 No abstract available.
Abstract
Background: An effective HIV vaccine will most likely need to have potent immunogenicity and broad cross-subtype coverage. The aim of the HIV Vaccine Trials Network (HVTN) 124 was to evaluate safety and immunogenicity of a unique polyvalent DNA-protein HIV vaccine with matching envelope (Env) immunogens.
Methods: HVTN 124 was a randomised, phase 1, placebo-controlled, double-blind study, including participants who were HIV seronegative and aged 18-50 years at low risk for infection. The DNA vaccine comprised five plasmids: four copies expressing Env gp120 (clades A, B, C, and AE) and one gag p55 (clade C). The protein vaccine included four DNA vaccine-matched GLA-SE-adjuvanted recombinant gp120 proteins. Participants were enrolled across six clinical sites in the USA and were randomly assigned to placebo or one of two vaccine groups (ie, prime-boost or coadministration) in a 5:1 ratio in part A and a 7:1 ratio in part B. Vaccines were delivered via intramuscular needle injection. The primary outcomes were safety and tolerability, assessed via frequency, severity, and attributability of local and systemic reactogenicity and adverse events, laboratory safety measures, and early discontinuations. Part A evaluated safety. Part B evaluated safety and immunogenicity of two regimens: DNA prime (administered at months 0, 1, and 3) with protein boost (months 6 and 8), and DNA-protein coadministration (months 0, 1, 3, 6, and 8). All randomly assigned participants who received at least one dose were included in the safety analysis. The study is registered with ClinicalTrials.gov (NCT03409276) and is closed to new participants.
Findings: Between April 19, 2018 and Feb 13, 2019, 60 participants (12 in part A [five men and seven women] and 48 in part B [21 men and 27 women]) were enrolled. All 60 participants received at least one dose, and 14 did not complete follow-up (six of 21 in the prime-boost group and eight of 21 in the coadminstration group). 11 clinical adverse events deemed by investigators as study-related occurred in seven of 48 participants in part B (eight of 21 in the prime-boost group and three of 21 in the coadministration group). Local reactogenicity in the vaccine groups was common, but the frequency and severity of reactogenicity signs or symptoms did not differ between the prime-boost and coadministration groups (eg, 20 [95%] of 21 in the prime-boost group vs 21 [100%] of 21 in the coadministration group had either local pain or tenderness of any severity [p=1·00], and seven [33%] vs nine [43%] had either erythema or induration [p=0·97]), nor did laboratory safety measures. There were no delayed-type hypersensitivity reactions or vasculitis or any severe clinical adverse events related to vaccination. The most frequently reported systemic reactogenicity symptoms in the active vaccine groups were malaise or fatigue (five [50%] of ten in part A and 17 [81%] of 21 in the prime-boost group vs 15 [71%] of 21 in the coadministration group in part B), headache (five [50%] and 18 [86%] vs 12 [57%]), and myalgia (four [40%] and 13 [62%] vs ten [48%]), mostly of mild or moderate severity.
Interpretation: Both vaccine regimens were safe, warranting evaluation in larger trials.
Funding: US National Institutes of Health and US National Institute of Allergy and Infectious Diseases.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests IF, NG, KES, JRH, SE, SCDR, DEM, DCM, GDT, LC, and SL report support for the present manuscript from National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS, and the HIV Vaccine Trials Network (HVTN) paid to their institutions. SCDR and DEM report support for the present manuscript from the Bill & Melinda Gates Foundation, and grants or contracts outside of the submitted work from Janssen, Sanofi, and Moderna. IF reports grants or contracts outside of the submitted work from Janssen, Gilead, Moderna, Pfizer, and Sanofi paid to his institution, consulting fees from Gilead and ViiV, and participation or payment from Janssen for participation on a data safety monitoring board or advisory board. JRH reports support for attending meetings or travel from the HVTN. MCK reports grants or contracts from NIAID outside of the submitted work and participation on a data safety monitoring board or advisory board for the Worcester HIV Vaccine. SL reports royalties or licences from Cosmic Bliss Sciences related to polyvalent DNA–protein HIV vaccine IP from the University of Massachusetts Medical School, Worcester, MA, USA, and reports stock ownership of Cosmic Bliss Sciences since 2023. All other authors declare no competing interests.
Figures

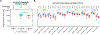
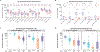

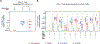
References
-
- UNAIDS. UNAIDS Fact Sheet 2022. 2021. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_... (accessed September 21 2022).
-
- Ugen KE, Nyland SB, Boyer JD, et al. DNA vaccination with HIV-1 expressing constructs elicits immune responses in humans. Vaccine 1998; 16(19): 1818–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

