Modulating extracellular matrix stiffness: a strategic approach to boost cancer immunotherapy
- PMID: 38693104
- PMCID: PMC11063215
- DOI: 10.1038/s41419-024-06697-4
Modulating extracellular matrix stiffness: a strategic approach to boost cancer immunotherapy
Abstract
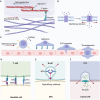
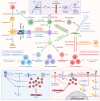
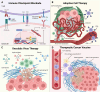
The interplay between extracellular matrix (ECM) stiffness and the tumor microenvironment is increasingly recognized as a critical factor in cancer progression and the efficacy of immunotherapy. This review comprehensively discusses the key factors regulating ECM remodeling, including the activation of cancer-associated fibroblasts and the accumulation and crosslinking of ECM proteins. Furthermore, it provides a detailed exploration of how ECM stiffness influences the behaviors of both tumor and immune cells. Significantly, the impact of ECM stiffness on the response to various immunotherapy strategies, such as immune checkpoint blockade, adoptive cell therapy, oncolytic virus therapy, and therapeutic cancer vaccines, is thoroughly examined. The review also addresses the challenges in translating research findings into clinical practice, highlighting the need for more precise biomaterials that accurately mimic the ECM and the development of novel therapeutic strategies. The insights offered aim to guide future research, with the potential to enhance the effectiveness of cancer immunotherapy modalities.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

