Macrophage depletion overcomes human hematopoietic cell engraftment failure in zebrafish embryo
- PMID: 38693109
- PMCID: PMC11063059
- DOI: 10.1038/s41419-024-06682-x
Macrophage depletion overcomes human hematopoietic cell engraftment failure in zebrafish embryo
Abstract
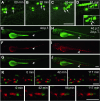
Zebrafish is widely adopted as a grafting model for studying human development and diseases. Current zebrafish xenotransplantations are performed using embryo recipients, as the adaptive immune system, responsible for host versus graft rejection, only reaches maturity at juvenile stage. However, transplanted primary human hematopoietic stem/progenitor cells (HSC) rapidly disappear even in zebrafish embryos, suggesting that another barrier to transplantation exists before the onset of adaptive immunity. Here, using a labelled macrophage zebrafish line, we demonstrated that engraftment of human HSC induces a massive recruitment of macrophages which rapidly phagocyte transplanted cells. Macrophages depletion, by chemical or pharmacological treatments, significantly improved the uptake and survival of transplanted cells, demonstrating the crucial implication of these innate immune cells for the successful engraftment of human cells in zebrafish. Beyond identifying the reasons for human hematopoietic cell engraftment failure, this work images the fate of human cells in real time over several days in macrophage-depleted zebrafish embryos.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
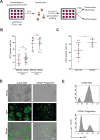


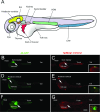
 Injection into the Duct of Cuvier (DC) of MO-buffer-treated embryos.
Injection into the Duct of Cuvier (DC) of MO-buffer-treated embryos.  Injection into the Duct of Cuvier (DC) of PU.1 Morphant embryos. F, G Transplantations of JK-GFP (F) and CD34-GFP (G) cells in the Duct of Cuvier (DC) of Lipo-C-treated embryos show a percentage of engraftment comparable to that obtained with injections into the yolk sac. Scale bar: 250 µm.
Injection into the Duct of Cuvier (DC) of PU.1 Morphant embryos. F, G Transplantations of JK-GFP (F) and CD34-GFP (G) cells in the Duct of Cuvier (DC) of Lipo-C-treated embryos show a percentage of engraftment comparable to that obtained with injections into the yolk sac. Scale bar: 250 µm.  Injection in the yolk sac of PBS-treated embryos.
Injection in the yolk sac of PBS-treated embryos.  Injection into the Duct of Cuvier (DC) of PBS-treated embryos.
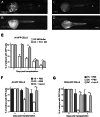
Injection into the Duct of Cuvier (DC) of PBS-treated embryos.  Injection into the Duct of Cuvier (DC) of Lipo-C-treated embryos. Data were presented as mean ± SD and are representative of 3 independent experiments with n = 15–20 (E), n = 10–30 (F) and n = 10–30 (G) embryos/group. (ns not significant or p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Injection into the Duct of Cuvier (DC) of Lipo-C-treated embryos. Data were presented as mean ± SD and are representative of 3 independent experiments with n = 15–20 (E), n = 10–30 (F) and n = 10–30 (G) embryos/group. (ns not significant or p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).References
Publication types
MeSH terms
Grants and funding
- PJA 20131200190/Fondation ARC pour la Recherche sur le Cancer (ARC Foundation for Cancer Research)
- ANR-14-CE11-0008/Agence Nationale de la Recherche (French National Research Agency)
- ANR-14-CE11-0008/Agence Nationale de la Recherche (French National Research Agency)
- ANR-14-CE11-0008/Agence Nationale de la Recherche (French National Research Agency)
- Ligue Régionale EST/Ligue Contre le Cancer
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

