Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids
- PMID: 38693114
- PMCID: PMC11063052
- DOI: 10.1038/s41419-024-06703-9
Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids
Abstract
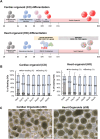
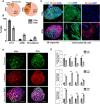
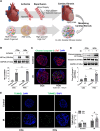
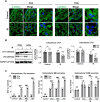
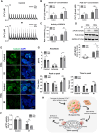
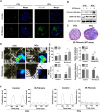
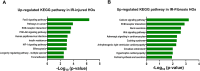
Heart disease involves irreversible myocardial injury that leads to high morbidity and mortality rates. Numerous cell-based cardiac in vitro models have been proposed as complementary approaches to non-clinical animal research. However, most of these approaches struggle to accurately replicate adult human heart conditions, such as myocardial infarction and ventricular remodeling pathology. The intricate interplay between various cell types within the adult heart, including cardiomyocytes, fibroblasts, and endothelial cells, contributes to the complexity of most heart diseases. Consequently, the mechanisms behind heart disease induction cannot be attributed to a single-cell type. Thus, the use of multi-cellular models becomes essential for creating clinically relevant in vitro cell models. This study focuses on generating self-organizing heart organoids (HOs) using human-induced pluripotent stem cells (hiPSCs). These organoids consist of cardiomyocytes, fibroblasts, and endothelial cells, mimicking the cellular composition of the human heart. The multi-cellular composition of HOs was confirmed through various techniques, including immunohistochemistry, flow cytometry, q-PCR, and single-cell RNA sequencing. Subsequently, HOs were subjected to hypoxia-induced ischemia and ischemia-reperfusion (IR) injuries within controlled culture conditions. The resulting phenotypes resembled those of acute myocardial infarction (AMI), characterized by cardiac cell death, biomarker secretion, functional deficits, alterations in calcium ion handling, and changes in beating properties. Additionally, the HOs subjected to IR efficiently exhibited cardiac fibrosis, displaying collagen deposition, disrupted calcium ion handling, and electrophysiological anomalies that emulate heart disease. These findings hold significant implications for the advancement of in vivo-like 3D heart and disease modeling. These disease models present a promising alternative to animal experimentation for studying cardiac diseases, and they also serve as a platform for drug screening to identify potential therapeutic targets.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

