Primary prophylaxis with mTOR inhibitor enhances T cell effector function and prevents heart transplant rejection during talimogene laherparepvec therapy of squamous cell carcinoma
- PMID: 38693123
- PMCID: PMC11063183
- DOI: 10.1038/s41467-024-47965-3
Primary prophylaxis with mTOR inhibitor enhances T cell effector function and prevents heart transplant rejection during talimogene laherparepvec therapy of squamous cell carcinoma
Abstract
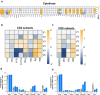
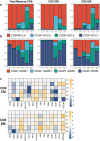
The application of mammalian target of rapamycin inhibition (mTORi) as primary prophylactic therapy to optimize T cell effector function while preserving allograft tolerance remains challenging. Here, we present a comprehensive two-step therapeutic approach in a male patient with metastatic cutaneous squamous cell carcinoma and heart transplantation followed with concomitant longitudinal analysis of systemic immunologic changes. In the first step, calcineurin inhibitor/ mycophenolic acid is replaced by the mTORi everolimus to achieve an improved effector T cell status with increased cytotoxic activity (perforin, granzyme), enhanced proliferation (Ki67) and upregulated activation markers (CD38, CD69). In the second step, talimogene laherparepvec (T-VEC) injection further enhances effector function by switching CD4 and CD8 cells from central memory to effector memory profiles, enhancing Th1 responses, and boosting cytotoxic and proliferative activities. In addition, cytokine release (IL-6, IL-18, sCD25, CCL-2, CCL-4) is enhanced and the frequency of circulating regulatory T cells is increased. Notably, no histologic signs of allograft rejection are observed in consecutive end-myocardial biopsies. These findings provide valuable insights into the dynamics of T cell activation and differentiation and suggest that timely initiation of mTORi-based primary prophylaxis may provide a dual benefit of revitalizing T cell function while maintaining allograft tolerance.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

