Edible mycelium as proliferation and differentiation support for anchorage-dependent animal cells in cultivated meat production
- PMID: 38693150
- PMCID: PMC11063153
- DOI: 10.1038/s41538-024-00263-0
Edible mycelium as proliferation and differentiation support for anchorage-dependent animal cells in cultivated meat production
Abstract
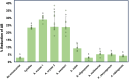
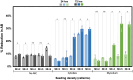
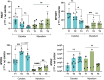
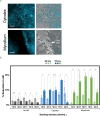
Cultivated meat production requires bioprocess optimization to achieve cell densities that are multiple orders of magnitude higher compared to conventional cell culture techniques. These processes must maximize resource efficiency and cost-effectiveness by attaining high cell growth productivity per unit of medium. Microcarriers, or carriers, are compatible with large-scale bioreactor use, and offer a large surface-area-to-volume ratio for the adhesion and proliferation of anchorage-dependent animal cells. An ongoing challenge persists in the efficient retrieval of cells from the carriers, with conflicting reports on the effectiveness of trypsinization and the need for additional optimization measures such as carrier sieving. To surmount this issue, edible carriers have been proposed, offering the advantage of integration into the final food product while providing opportunities for texture, flavor, and nutritional incorporation. Recently, a proof of concept (POC) utilizing inactivated mycelium biomass derived from edible filamentous fungus demonstrated its potential as a support structure for myoblasts. However, this POC relied on a model mammalian cell line combination with a single mycelium species, limiting realistic applicability to cultivated meat production. This study aims to advance the POC. We found that the species of fungi composing the carriers impacts C2C12 myoblast cell attachment-with carriers derived from Aspergillus oryzae promoting the best proliferation. C2C12 myoblasts effectively differentiated on mycelium carriers when induced in myogenic differentiation media. Mycelium carriers also supported proliferation and differentiation of bovine satellite cells. These findings demonstrate the potential of edible mycelium carrier technology to be readily adapted in product development within the cultivated meat industry.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests but the following competing non-financial Interests: Minami Ogawa is cofounder of Optimized Foods. The company did not affect the authenticity and objectivity of the experimental results of this work.
Figures

Similar articles
-
Emulsion-templated microparticles with tunable stiffness and topology: Applications as edible microcarriers for cultured meat.Biomaterials. 2022 Aug;287:121669. doi: 10.1016/j.biomaterials.2022.121669. Epub 2022 Jul 12. Biomaterials. 2022. PMID: 35853359 Free PMC article.
-
Assessing Edible Filamentous Fungal Carriers as Cell Supports for Growth of Yeast and Cultivated Meat.Foods. 2022 Oct 9;11(19):3142. doi: 10.3390/foods11193142. Foods. 2022. PMID: 36230217 Free PMC article.
-
Microcarriers for Upscaling Cultured Meat Production.Front Nutr. 2020 Feb 20;7:10. doi: 10.3389/fnut.2020.00010. eCollection 2020. Front Nutr. 2020. PMID: 32154261 Free PMC article. Review.
-
Development of cultivable alginate fibers for an ideal cell-cultivated meat scaffold and production of hybrid cultured meat.Carbohydr Polym. 2023 Dec 1;321:121287. doi: 10.1016/j.carbpol.2023.121287. Epub 2023 Aug 11. Carbohydr Polym. 2023. PMID: 37739499
-
Systems biology and metabolic modeling for cultivated meat: A promising approach for cell culture media optimization and cost reduction.Compr Rev Food Sci Food Saf. 2023 Jul;22(4):3422-3443. doi: 10.1111/1541-4337.13193. Epub 2023 Jun 12. Compr Rev Food Sci Food Saf. 2023. PMID: 37306528 Review.
Cited by
-
Animal-free edible scaffolds from soy protein isolate for the scalable production of cultured meat.Curr Res Food Sci. 2025 Jun 26;11:101129. doi: 10.1016/j.crfs.2025.101129. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40689295 Free PMC article.
-
Evaluation of the Protein Profile of a Saccharomyces cerevisiae Strain Immobilized in Biocapsules for Use in Fermented Foods.Foods. 2024 Nov 29;13(23):3871. doi: 10.3390/foods13233871. Foods. 2024. PMID: 39682943 Free PMC article.
-
Filamentous fungal pellets as versatile platforms for cell immobilization: developments to date and future perspectives.Microb Cell Fact. 2024 Oct 16;23(1):280. doi: 10.1186/s12934-024-02554-3. Microb Cell Fact. 2024. PMID: 39415192 Free PMC article. Review.
-
Review on mushroom mycelium-based products and their production process: from upstream to downstream.Bioresour Bioprocess. 2025 Jan 10;12(1):3. doi: 10.1186/s40643-024-00836-7. Bioresour Bioprocess. 2025. PMID: 39794674 Free PMC article. Review.
-
Engineering cell-derived extracellular matrix for peripheral nerve regeneration.Mater Today Bio. 2024 Jun 13;27:101125. doi: 10.1016/j.mtbio.2024.101125. eCollection 2024 Aug. Mater Today Bio. 2024. PMID: 38979129 Free PMC article. Review.
References
-
- Moritz MSM, Verbruggen SEL, Post MJ. Alternatives for large-scale production of cultured beef: a review. J. Integr. Agric. 2015;14:208–216. doi: 10.1016/S2095-3119(14)60889-3. - DOI
-
- Post, M. & van der Weele, C. Principles of tissue engineering for food. Principles of Tissue Engineering 1647–1662 10.1016/B978-0-12-398358-9.00078-1 (Elsevier, 2014).
-
- Rowley, J., Abraham, E., Campbell, A., Brandwein, H. & Oh, S. Meeting lot-size challenges of manufacturing adherent cells for therapy. J. BioProcess Int.10, 16–22 (2012).

