Mitochondrial transfer mediates endothelial cell engraftment through mitophagy
- PMID: 38693258
- PMCID: PMC11574736
- DOI: 10.1038/s41586-024-07340-0
Mitochondrial transfer mediates endothelial cell engraftment through mitophagy
Abstract
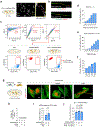
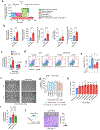
Ischaemic diseases such as critical limb ischaemia and myocardial infarction affect millions of people worldwide1. Transplanting endothelial cells (ECs) is a promising therapy in vascular medicine, but engrafting ECs typically necessitates co-transplanting perivascular supporting cells such as mesenchymal stromal cells (MSCs), which makes clinical implementation complicated2,3. The mechanisms that enable MSCs to facilitate EC engraftment remain elusive. Here we show that, under cellular stress, MSCs transfer mitochondria to ECs through tunnelling nanotubes, and that blocking this transfer impairs EC engraftment. We devised a strategy to artificially transplant mitochondria, transiently enhancing EC bioenergetics and enabling them to form functional vessels in ischaemic tissues without the support of MSCs. Notably, exogenous mitochondria did not integrate into the endogenous EC mitochondrial pool, but triggered mitophagy after internalization. Transplanted mitochondria co-localized with autophagosomes, and ablation of the PINK1-Parkin pathway negated the enhanced engraftment ability of ECs. Our findings reveal a mechanism that underlies the effects of mitochondrial transfer between mesenchymal and endothelial cells, and offer potential for a new approach for vascular cell therapy.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures













Comment in
-
Artificially transplanted mitochondria in endothelial cells promote mitophagy.Nat Rev Cardiol. 2024 Jul;21(7):439. doi: 10.1038/s41569-024-01041-x. Nat Rev Cardiol. 2024. PMID: 38773356 No abstract available.
References
-
- Beckman JA, Schneider PA & Conte MS Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ. Res 128, 1885–1912 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

