Geographic variation of mutagenic exposures in kidney cancer genomes
- PMID: 38693263
- PMCID: PMC11111402
- DOI: 10.1038/s41586-024-07368-2
Geographic variation of mutagenic exposures in kidney cancer genomes
Abstract
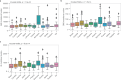
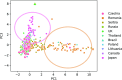
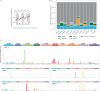
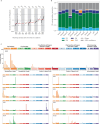
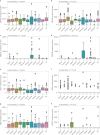
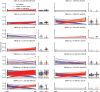
International differences in the incidence of many cancer types indicate the existence of carcinogen exposures that have not yet been identified by conventional epidemiology make a substantial contribution to cancer burden1. In clear cell renal cell carcinoma, obesity, hypertension and tobacco smoking are risk factors, but they do not explain the geographical variation in its incidence2. Underlying causes can be inferred by sequencing the genomes of cancers from populations with different incidence rates and detecting differences in patterns of somatic mutations. Here we sequenced 962 clear cell renal cell carcinomas from 11 countries with varying incidence. The somatic mutation profiles differed between countries. In Romania, Serbia and Thailand, mutational signatures characteristic of aristolochic acid compounds were present in most cases, but these were rare elsewhere. In Japan, a mutational signature of unknown cause was found in more than 70% of cases but in less than 2% elsewhere. A further mutational signature of unknown cause was ubiquitous but exhibited higher mutation loads in countries with higher incidence rates of kidney cancer. Known signatures of tobacco smoking correlated with tobacco consumption, but no signature was associated with obesity or hypertension, suggesting that non-mutagenic mechanisms of action underlie these risk factors. The results of this study indicate the existence of multiple, geographically variable, mutagenic exposures that potentially affect tens of millions of people and illustrate the opportunities for new insights into cancer causation through large-scale global cancer genomics.
© 2024. The Author(s).
Conflict of interest statement
L.B.A. is a compensated consultant and has equity interest in io9 and Genome Insight. His spouse is an employee of Biotheranostics. L.B.A. is also an inventor of a US Patent 10,776,718 for source identification by non-negative matrix factorization. E.N.B. and L.B.A. declare US provisional patent applications with serial numbers: 63/289,601, 63/269,033 and 63/483,237. L.B.A. also declares US provisional patent applications with serial numbers: 63/366,392, 63/367,846, 63/412,835 and 63/492,348. V.M. received honoraria from Ipsen, Bayer, AstraZeneca, Janssen, Astellas Pharm and MSD, and provided expert testimony to BMS, Bayer, MSD and Janssen. The other authors declare no competing interests.
Figures









Comment in
-
Mutational signatures of ccRCC vary between geographical regions.Nat Rev Nephrol. 2024 Jul;20(7):429. doi: 10.1038/s41581-024-00855-9. Nat Rev Nephrol. 2024. PMID: 38822189 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

