Hypoxia-inducible factor 3α1 increases epithelial-to-mesenchymal transition and iron uptake to drive colorectal cancer liver metastasis
- PMID: 38693428
- PMCID: PMC11183190
- DOI: 10.1038/s41416-024-02699-3
Hypoxia-inducible factor 3α1 increases epithelial-to-mesenchymal transition and iron uptake to drive colorectal cancer liver metastasis
Abstract
Background/objectives: Hypoxia-inducible factor (HIF)-3α1's role in colorectal cancer (CRC) cells, especially its effects on epithelial-mesenchymal transition (EMT), zinc finger E-box binding homeobox 2 (ZEB2) gene expression, and iron metabolism, remains largely unstudied. This research sought to elucidate these relationships.
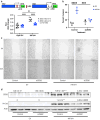
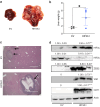
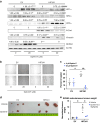
Methods: RNA-seq was conducted to investigate the impact of HIF-3α1 overexpression in CRC cells. Dual-luciferase reporter assays assessed the direct targeting of ZEB2 by HIF-3α1. Scratch assays measured changes in cell migration following HIF-3α1 overexpression and ZEB2 knockdown. The effects of HIF-3α1 overexpression on colon tumour growth and liver metastasis were examined in vivo. Iron chelation was used to explore the role of iron metabolism in HIF-3α1-mediated EMT and tumour growth.
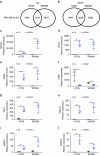
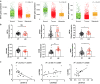
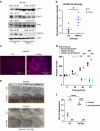
Results: HIF-3α1 overexpression induced EMT and upregulated ZEB2 expression, enhancing cancer cell migration. ZEB2 knockdown reduced mesenchymal markers and cell migration. HIF-3α1 promoted colon tumour growth and liver metastasis, increased transferrin receptor (TFRC) expression and cellular iron levels, and downregulated HIF-1α, HIF-2α, and NDRG1. Iron chelation mitigated HIF-3α1-mediated EMT, tumour growth, and survival.
Conclusions: HIF-3α1 plays a critical role in colon cancer progression by promoting EMT, iron accumulation, and metastasis through ZEB2 and TFRC regulation, suggesting potential therapeutic targets in CRC.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Manfredi S, Lepage C, Hatem C, Coatmeyr O, Faivre J, Bouvier A. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9. doi: 10.1097/01.sla.0000217629.94941.cf. - DOI - PMC - PubMed
-
- Leith JT, Padfield G, Faulkner L, Michelson S. Hypoxic fractions in xenografted human colon tumors. Cancer Res. 1991;51:5139–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

