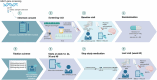
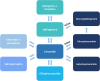
Study protocol of the GRoningen early-PD Ambroxol treatment (GREAT) trial: a randomized, double-blind, placebo-controlled, single center trial with ambroxol in Parkinson patients with a GBA mutation
- PMID: 38693511
- PMCID: PMC11061939
- DOI: 10.1186/s12883-024-03629-9
Study protocol of the GRoningen early-PD Ambroxol treatment (GREAT) trial: a randomized, double-blind, placebo-controlled, single center trial with ambroxol in Parkinson patients with a GBA mutation
Abstract
Background: To date, no disease modifying therapies are available for Parkinson's disease (PD). Since PD is the second most prevalent neurodegenerative disorder, there is a high demand for such therapies. Both environmental and genetic risk factors play an important role in the etiology and progression of PD. The most common genetic risk factor for PD is a mutation in the GBA1(GBA)-gene, encoding the lysosomal enzyme glucocerebrosidase (GCase). The mucolytic ambroxol is a repurposed drug, which has shown the property to upregulate GCase activity in-vitro and in-vivo. Ambroxol therefore has the potency to become a disease modifying therapy in PD, which was the reason to design this randomized controlled trial with ambroxol in PD patients.
Methods: This trial is a single-center, double-blind, randomized, placebo-controlled study, including 80 PD patients with a GBA mutation, receiving either ambroxol 1800 mg/day or placebo for 48 weeks. The primary outcome measure is the Unified Parkinson's Disease Rating Scale motor subscore (part III) of the Movement Disorder Society (MDS-UPDRSIII) in the practically defined off-state at 60 weeks (after a 12-week washout period). Secondary outcomes include a 3,4-dihydroxy-6-18F-fluoro-I-phenylalanine ([18F]FDOPA) PET-scan of the brain, Magnetic Resonance Imaging (with resting state f-MRI and Diffusion Tensor Imaging), GCase activity, both intra- and extracellularly, sphingolipid profiles in plasma, Montreal Cognitive Assessment (MoCA), quality of life (QoL) measured by the Parkinson's Disease Questionnaire (PDQ-39) and the Non-Motor Symptom Scale (NMSS) questionnaire.
Discussion: Ambroxol up to 1200 mg/day has shown effects on human cerebrospinal fluid endpoints, which supports at least passage of the blood-brain-barrier. The dose titration in this trial up to 1800 mg/day will reveal if this dose level is safe and also effective in modifying the course of the disease.
Trial registration: NCT05830396. Registration date: March 20, 2023.
Keywords: Ambroxol; Disease modifying therapy; GBA1; Glucocerebrosidase; Interventional study; Parkinson’s disease.
© 2024. The Author(s).
Conflict of interest statement
TvL has received grant support from the MJFF, the UMCG, Menzis, Weston Brain Institute and the Dutch Brain Foundation. Consultancy fees were received from AbbVie, Britannia Pharm., Centrapharm and Neuroderm. Speaker fees were received from AbbVie, Britannia Pharm. and Eurocept.
The remaining authors declare that they have no known competing interests.
Figures
Similar articles
-
Ambroxol as a novel disease-modifying treatment for Parkinson's disease dementia: protocol for a single-centre, randomized, double-blind, placebo-controlled trial.BMC Neurol. 2019 Feb 9;19(1):20. doi: 10.1186/s12883-019-1252-3. BMC Neurol. 2019. PMID: 30738426 Free PMC article. Clinical Trial.
-
Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial.JAMA Neurol. 2020 Apr 1;77(4):427-434. doi: 10.1001/jamaneurol.2019.4611. JAMA Neurol. 2020. PMID: 31930374 Free PMC article. Clinical Trial.
-
LC-MS/MS-Based Determination of Ambroxol in Human Plasma and Cerebrospinal Fluid: Validation and Applicability in a Phase II Study on GBA-Associated Parkinson's Disease Patients.Int J Mol Sci. 2025 Jun 25;26(13):6094. doi: 10.3390/ijms26136094. Int J Mol Sci. 2025. PMID: 40649871 Free PMC article. Clinical Trial.
-
Combined beta-glucosylceramide and ambroxol hydrochloride in patients with Gaucher related Parkinson disease: From clinical observations to drug development.Blood Cells Mol Dis. 2018 Feb;68:117-120. doi: 10.1016/j.bcmd.2016.10.028. Epub 2016 Nov 12. Blood Cells Mol Dis. 2018. PMID: 27866808 Review.
-
Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues.Pharmacol Ther. 2023 Jun;246:108419. doi: 10.1016/j.pharmthera.2023.108419. Epub 2023 Apr 19. Pharmacol Ther. 2023. PMID: 37080432 Review.
Cited by
-
Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson's disease.Transl Neurodegener. 2024 Sep 12;13(1):48. doi: 10.1186/s40035-024-00437-6. Transl Neurodegener. 2024. PMID: 39267121 Free PMC article. Review.
-
Microglial dynamics and neuroinflammation in prodromal and early Parkinson's disease.J Neuroinflammation. 2025 May 21;22(1):136. doi: 10.1186/s12974-025-03462-y. J Neuroinflammation. 2025. PMID: 40399949 Free PMC article. Review.
References
-
- Barker LA, Morton N, Morrison TG, Mcguire BE. Inter-rater reliability of the Dysexecutive questionnaire (DEX): comparative data from non-clinician respondents - all raters are not equal. Brain Inj. 2011;25(10):997–1004. - PubMed
-
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA - J Am Med Assoc. 2020;323(6):548–560. - PubMed
-
- Dawson VL, Dawson TM. Promising disease-modifying therapies for Parkinson ’ s disease. Sci Transl Med. 2019;1659:1–4. - PubMed
-
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous