The potential and promise for clinical application of adoptive T cell therapy in cancer
- PMID: 38693513
- PMCID: PMC11064426
- DOI: 10.1186/s12967-024-05206-7
The potential and promise for clinical application of adoptive T cell therapy in cancer
Abstract
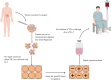
Adoptive cell therapy has revolutionized cancer treatment, especially for hematologic malignancies. T cells are the most extensively utilized cells in adoptive cell therapy. Currently, tumor-infiltrating lymphocytes, T cell receptor-transgenic T cells and chimeric antigen receptor T cells are the three main adoptive T cell therapies. Tumor-infiltrating lymphocytes kill tumors by reinfusing enlarged lymphocytes that naturally target tumor-specific antigens into the patient. T cell receptor-transgenic T cells have the ability to specifically destroy tumor cells via the precise recognition of exogenous T cell receptors with major histocompatibility complex. Chimeric antigen receptor T cells transfer genes with specific antigen recognition structural domains and T cell activation signals into T cells, allowing T cells to attack tumors without the assistance of major histocompatibility complex. Many barriers have been demonstrated to affect the clinical efficacy of adoptive T cell therapy, such as tumor heterogeneity and antigen loss, hard trafficking and infiltration, immunosuppressive tumor microenvironment and T cell exhaustion. Several strategies to improve the efficacy of adoptive T cell therapy have been explored, including multispecific chimeric antigen receptor T cell therapy, combination with immune checkpoint blockade, targeting the immunosuppressive tumor microenvironment, etc. In this review, we will summarize the current status and clinical application, followed by major bottlenecks in adoptive T cell therapy. In addition, we will discuss the promising strategies to improve adoptive T cell therapy. Adoptive T cell therapy will result in even more incredible advancements in solid tumors if the aforementioned problems can be handled.
Keywords: Adoptive cell therapy; Chimeric antigen receptor; Immunotherapy; T cell receptor; Tumor-infiltrating lymphocytes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
[Adoptive cell therapy for solid tumors].Rinsho Ketsueki. 2024;65(7):652-661. doi: 10.11406/rinketsu.65.652. Rinsho Ketsueki. 2024. PMID: 39098016 Review. Japanese.
-
Advanced Strategies of CAR-T Cell Therapy in Solid Tumors and Hematological Malignancies.Recent Pat Anticancer Drug Discov. 2024;19(5):557-572. doi: 10.2174/0115748928277331231218115402. Recent Pat Anticancer Drug Discov. 2024. PMID: 38213150 Review.
-
CAR-T therapy in solid tumors.Cancer Cell. 2025 Apr 14;43(4):665-679. doi: 10.1016/j.ccell.2025.03.019. Cancer Cell. 2025. PMID: 40233718 Review.
-
Tumor-Infiltrating Lymphocytes, CAR-, and T-Cell Receptor-Modified T Cells in Solid Cancer Oncology.Oncol Res Treat. 2025;48(5):294-304. doi: 10.1159/000543998. Epub 2025 Feb 12. Oncol Res Treat. 2025. PMID: 39938499 Review.
-
Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors.Mol Cancer. 2018 Jan 12;17(1):7. doi: 10.1186/s12943-018-0759-3. Mol Cancer. 2018. PMID: 29329591 Free PMC article. Review.
Cited by
-
Diverse approaches and sources to derive antitumor T cell for liver cancer: a single-cell sequence based research.Hepatol Int. 2025 Apr 7. doi: 10.1007/s12072-025-10818-2. Online ahead of print. Hepatol Int. 2025. PMID: 40193035
-
Harnessing the tumor microenvironment: targeted cancer therapies through modulation of epithelial-mesenchymal transition.J Hematol Oncol. 2025 Jan 13;18(1):6. doi: 10.1186/s13045-024-01634-6. J Hematol Oncol. 2025. PMID: 39806516 Free PMC article. Review.
-
Unlocking the potential: advancements and applications of gene therapy in severe disorders.Ann Med. 2025 Dec;57(1):2516697. doi: 10.1080/07853890.2025.2516697. Epub 2025 Jun 17. Ann Med. 2025. PMID: 40526097 Free PMC article. Review.
-
Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications.Signal Transduct Target Ther. 2024 Nov 14;9(1):322. doi: 10.1038/s41392-024-02002-z. Signal Transduct Target Ther. 2024. PMID: 39543114 Free PMC article. Review.
-
Targeting cancer with precision: strategical insights into TCR-engineered T cell therapies.Theranostics. 2025 Jan 1;15(1):300-323. doi: 10.7150/thno.104594. eCollection 2025. Theranostics. 2025. PMID: 39744228 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

