A systematic review on reporting quality of economic evaluations for negotiated glucose-lowering drugs in China national reimbursement drug list
- PMID: 38693514
- PMCID: PMC11064232
- DOI: 10.1186/s12913-024-11001-3
A systematic review on reporting quality of economic evaluations for negotiated glucose-lowering drugs in China national reimbursement drug list
Abstract
Background: This study aimed to examine the reporting quality of existing economic evaluations for negotiated glucose-lowering drugs (GLDs) included in China National Reimbursement Drug List (NRDL) using the Consolidated Health Economic Evaluation Reporting Standards 2013 (CHEERS 2013).
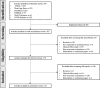
Methods: We performed a systematic literature research through 7 databases to identify published economic evaluations for GLDs included in the China NRDL up to March 2021. Reporting quality of identified studies was assessed by two independent reviewers based on the CHEERS checklist. The Kruskal-Wallis test and Mann-Whitney U test were performed to examine the association between reporting quality and characteristics of the identified studies.
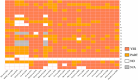
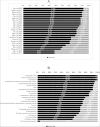
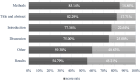
Results: We have identified 24 studies, which evaluated six GLDs types. The average score rate of the included studies was 77.41% (SD:13.23%, Range 47.62%-91.67%). Among all the required reporting items, characterizing heterogeneity (score rate = 4.17%) was the least satisfied item. Among six parts of CHEERS, results part scored least at 0.55 (score rate = 54.79%) because of the incompleteness of characterizing uncertainty. Results from the Kruskal-Wallis test and Mann-Whitney U test showed that model choice, journal type, type of economic evaluations, and study perspective were associated with the reporting quality of the studies.
Conclusions: There remains room to improve the reporting quality of economic evaluations for GLDs in NRDL. Checklists such as CHEERS should be widely used to improve the reporting quality of economic researches in China.
Keywords: Economic evaluations; Glucose-lowering drugs; National Reimbursement Drug List (NRDL); Quality evaluation; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Were economic evaluations well reported for the newly listed oncology drugs in China's national reimbursement drug list.BMC Health Serv Res. 2022 Dec 3;22(1):1475. doi: 10.1186/s12913-022-08858-7. BMC Health Serv Res. 2022. PMID: 36463141 Free PMC article.
-
Reporting quality of economic evaluations of the negotiated Traditional Chinese Medicines in national reimbursement drug list of China: A systematic review.Integr Med Res. 2023 Mar;12(1):100915. doi: 10.1016/j.imr.2022.100915. Epub 2022 Dec 23. Integr Med Res. 2023. PMID: 36632129 Free PMC article.
-
Systematic review of reporting quality of economic evaluations in plastic surgery based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.J Plast Reconstr Aesthet Surg. 2021 Oct;74(10):2458-2466. doi: 10.1016/j.bjps.2021.05.017. Epub 2021 Jun 21. J Plast Reconstr Aesthet Surg. 2021. PMID: 34217645
-
Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force.Value Health. 2013 Mar-Apr;16(2):231-50. doi: 10.1016/j.jval.2013.02.002. Value Health. 2013. PMID: 23538175
-
Sponsorship bias in published pharmacoeconomic evaluations of national reimbursement negotiation drugs in China: a systematic review.BMJ Glob Health. 2023 Nov 29;8(11):e012780. doi: 10.1136/bmjgh-2023-012780. BMJ Glob Health. 2023. PMID: 38030227 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

