Campylobacter jejuni virulence factors: update on emerging issues and trends
- PMID: 38693534
- PMCID: PMC11064354
- DOI: 10.1186/s12929-024-01033-6
Campylobacter jejuni virulence factors: update on emerging issues and trends
Abstract
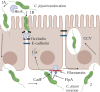
Campylobacter jejuni is a very common cause of gastroenteritis, and is frequently transmitted to humans through contaminated food products or water. Importantly, C. jejuni infections have a range of short- and long-term sequelae such as irritable bowel syndrome and Guillain Barre syndrome. C. jejuni triggers disease by employing a range of molecular strategies which enable it to colonise the gut, invade the epithelium, persist intracellularly and avoid detection by the host immune response. The objective of this review is to explore and summarise recent advances in the understanding of the C. jejuni molecular factors involved in colonisation, invasion of cells, collective quorum sensing-mediated behaviours and persistence. Understanding the mechanisms that underpin the pathogenicity of C. jejuni will enable future development of effective preventative approaches and vaccines against this pathogen.
Keywords: Campylobacter jejuni; Biofilm; Chemotaxis; Pathogenesis; Polysaccharide capsule; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
Authors declare no competing interest.
Figures




References
-
- Chantzaras A-P, Karageorgos S, Panagiotou P, Georgiadou E, Chousou T, Spyridopoulou K, Paradeisis G, Kanaka-Gantenbein C, Botsa E. Myocarditis in a pediatric patient with Campylobacter enteritis: a case report and literature review. Trop Med Infect Dis. 2021;6(4):212. doi: 10.3390/tropicalmed6040212. - DOI - PMC - PubMed
-
- Dessouky YE, Elsayed SW, Abdelsalam NA, Saif NA, Álvarez-Ordóñez A, Elhadidy M. Genomic insights into zoonotic transmission and antimicrobial resistance in Campylobacter jejuni from farm to fork: a one health perspective. Gut Pathog. 2022;14(1):44. doi: 10.1186/s13099-022-00517-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

